SOS1 and PTPN11 Mutations in Five Cases of Noonan Syndrome with Multiple Giant Cell Lesions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
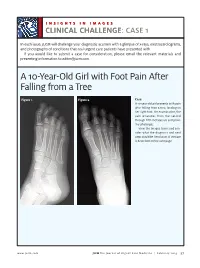
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -
Another Family with the 'Habsburg Jaw'
J Med Genet: first published as 10.1136/jmg.25.12.838 on 1 December 1988. Downloaded from Journal of Medical Genetics 1988, 25, 838-842 Another family with the 'Habsburg jaw' E M THOMPSON* AND R M WINTERt From *the Department of Paediatric Genetics, Institute of Child Health, 30 Guilford Street, London WCIN IEH; and tthe Kennedy-Galton Centre, Clinical Research Centre, Northwick Park Hospital, Watford Road, Harrow, Middlesex HAI 3HJ. SUMMARY We report a three generation family with similar facial characteristics to those of the Royal Habsburgs, including mandibular prognathism, thickened lower lip, prominent, often misshapen nose, flat malar areas, and mildly everted lower eyelids. One child had craniosyn- ostosis which may be part of the syndrome. The Habsburgs, one of Europe's foremost royal nine successive generations of the family' (fig 1). families, are famous not only for the duration of Although it was transmitted as an autosomal their reign and brilliance of their leadership, but also dominant trait, males were more severely affected because they represent one of the few examples of than females. Examination of the abundant portraits Mendelian inheritance of facial characteristics. This of the family shows, in addition to prognathism, a has been referred to as the 'Habsburg jaw' to thick, everted lower lip, a large, often misshar -n describe the prognathic mandible which was seen in nose with a prominent dorsal hump, a tendenc) to flattening of the malar areas, and mild eversior of Received for publication 9 December 1987. copyright. Revised version accepted for publication 2 March 1988. http://jmg.bmj.com/ on October 3, 2021 by guest. -

Intersectin 1 Forms Complexes with SGIP1 and Reps1 in Clathrin-Coated
Biochemical and Biophysical Research Communications 402 (2010) 408–413 Contents lists available at ScienceDirect Biochemical and Biophysical Research Communications journal homepage: www.elsevier.com/locate/ybbrc Intersectin 1 forms complexes with SGIP1 and Reps1 in clathrin-coated pits ⇑ Oleksandr Dergai a, ,1, Olga Novokhatska a,1, Mykola Dergai a, Inessa Skrypkina a, Liudmyla Tsyba a, Jacques Moreau b, Alla Rynditch a a Department of Functional Genomics, Institute of Molecular Biology and Genetics, NASU, 150 Zabolotnogo Street, 03680 Kyiv, Ukraine b Molecular Mechanisms of Development, Jacques Monod Institute, Development and Neurobiology Program, UMR7592 CNRS – Paris Diderot University, 15 rue Hélène Brion, 75205 Paris Cedex 13, France article info abstract Article history: Intersectin 1 (ITSN1) is an evolutionarily conserved adaptor protein involved in clathrin-mediated endo- Received 7 October 2010 cytosis, cellular signaling and cytoskeleton rearrangement. ITSN1 gene is located on human chromosome Available online 12 October 2010 21 in Down syndrome critical region. Several studies confirmed role of ITSN1 in Down syndrome pheno- type. Here we report the identification of novel interconnections in the interaction network of this endo- Keywords: cytic adaptor. We show that the membrane-deforming protein SGIP1 (Src homology 3-domain growth Endocytosis factor receptor-bound 2-like (endophilin) interacting protein 1) and the signaling adaptor Reps1 (RalBP Adaptor proteins associated Eps15-homology domain protein) interact with ITSN1 in vivo. Both interactions are mediated Intersectin 1 by the SH3 domains of ITSN1 and proline-rich motifs of protein partners. Moreover complexes compris- Protein interactions SGIP1 ing SGIP1, Reps1 and ITSN1 have been identified. We also identified new interactions between SGIP1, Reps1 Reps1 and the BAR (Bin/amphiphysin/Rvs) domain-containing protein amphiphysin 1. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Tutankhamun's Dentition: the Pharaoh and His Teeth
Brazilian Dental Journal (2015) 26(6): 701-704 ISSN 0103-6440 http://dx.doi.org/10.1590/0103-6440201300431 1Department of Oral and Maxillofacial Tutankhamun’s Dentition: Surgery, University Hospital of Leipzig, Leipzig, Germany The Pharaoh and his Teeth 2Institute of Egyptology/Egyptian Museum Georg Steindorff, University of Leipzig, Leipzig, Germany 3Department of Orthodontics, University Hospital of Greifswald, Greifswald, Germany Niels Christian Pausch1, Franziska Naether2, Karl Friedrich Krey3 Correspondence: Dr. Niels Christian Pausch, Liebigstraße 12, 04103 Leipzig, Germany. Tel: +49- 341-97-21160. e-mail: niels. [email protected] Tutankhamun was a Pharaoh of the 18th Dynasty (New Kingdom) in ancient Egypt. Medical and radiological investigations of his skull revealed details about the jaw and teeth status of the mummy. Regarding the jaw relation, a maxillary prognathism, a mandibular retrognathism and micrognathism have been discussed previously. A cephalometric analysis was performed using a lateral skull X-ray and a review of the literature regarding Key Words: Tutankhamun’s King Tutankhamun´s mummy. The results imply diagnosis of mandibular retrognathism. dentition, cephalometric analysis, Furthermore, third molar retention and an incomplete, single cleft palate are present. mandibular retrognathism Introduction also been discussed (11). In 1922, the British Egyptologist Howard Carter found the undisturbed mummy of King Tutankhamun. The Case Report spectacular discovery enabled scientists of the following In the evaluation of Tutankhamun’s dentition and jaw decades to analyze the Pharaoh's remains. The mummy alignment, contemporary face reconstructions and coeval underwent multiple autopsies. Until now, little was artistic images can be of further use. However, the ancient published about the jaw and dentition of the King. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Mandibular Prognathism with Unilateral Crossbite—Treatment
message board Mandibular Prognathism with Unilateral Crossbite—Treatment Not Going Well Townie “3MOrtho” wonders how to treat a young boy with a slew of problems such as mandibular prognathism and a mild protrusion of the mandibular incisors 3Mortho Member Since: 11/18/07 Introduction: Post: 1 of 11 The patient is a 10-year-old boy with mandibular prognathism, unilateral crossbite, spacing in the lower arch, minimal crowding in the upper arch, skeletal Class III, and mild protrusion of mandibular incisors. The treatment was started with Delaire mask and RPE. At the end of this treatment (9 months) he had a tete-a-tete bite in the front and the upper arch was successfully expanded. Treatment was continued with Class III and open bite elastics, but the patient is not compliant in wearing the elastics. His second year in treatment, there is deviation of the mandible to the right and the bite cannot be closed with elastics. How would you proceed with the treatment? Would you extract in this case? n 14 DECEMBER 2017 // orthotown.com message board 10/4/2017 Fenrisúlfr Member Since: 02/25/09 What was the rationale for the early intervention/Phase 1? Any discussion re: surgery? Given his Post: 2 of 11 age, and pending a shift, the Class III is likely to significantly worsen with continued mand. growth. A prudent option may be to correct the transverse completely, alleviate any slides and then remove appliances. Once there has been cessation of growth, he can then be evaluated for surgical correction. n 10/4/2017 Shwan Member Since: 08/02/10 There is skeletal mandibular asymmetry that will worsen with time. -

Restriction in Infants
Introduction Ankyloglossia is a congenital anomaly characterized by presence of a hypertrophic lingual frenulum which is short and attached to the very tip of the tongue, limiting its normal movements. Clinically the patient cannot protrude the tongue past the incisal edge of the gingiva and the tongue becomes heart shaped when attempted to protrude. The restricted movements of the tongue can result in problems with breast feeding, lactation, speech disorders and other oral motor disorders like problems with swallowing and licking.1,2 The clinical significance of this anomaly and symptoms produced and the best method of management have been the subject of debate for some time. A review of the relation between the above problems and ankyloglossia is presented. Review Tongue is an accessory organ of importance in mastication, deglutition and speech; many authors describe its stimulatory influence on the development of the dental arches.3 At birth, the tongue unconfined by the teeth, extends outward between the maxillary and mandibular occlusal gum pads. The “infantile swallow” with jaws parted and tongue placed between the jaws is replaced by the adult swallow at the age of two and half years of age. At the start of the normal adult swallow, the lips are closed, the teeth are brought into full occlusion, and the tip is raised and placed against the anterior portion of the palate. The respiratory opening, the nasal cavity, and the anterior portion of the mouth are all sealed off as the tongue, in a sweeping and undulating motion, sweeps backward the mass of chewed food.3,4 The tongue is always short at birth and the tip of the tongue is yet incompletely developed. -

Phenotypic and Genotypic Characterisation of Noonan-Like
1of5 ELECTRONIC LETTER J Med Genet: first published as 10.1136/jmg.2004.024091 on 2 February 2005. Downloaded from Phenotypic and genotypic characterisation of Noonan-like/ multiple giant cell lesion syndrome J S Lee, M Tartaglia, B D Gelb, K Fridrich, S Sachs, C A Stratakis, M Muenke, P G Robey, M T Collins, A Slavotinek ............................................................................................................................... J Med Genet 2005;42:e11 (http://www.jmedgenet.com/cgi/content/full/42/2/e11). doi: 10.1136/jmg.2004.024091 oonan-like/multiple giant cell lesion syndrome (NL/ MGCLS; OMIM 163955) is a rare condition1–3 with Key points Nphenotypic overlap with Noonan’s syndrome (OMIM 163950) and cherubism (OMIM 118400) (table 1). N Noonan-like/multiple giant cell lesion syndrome (NL/ Recently, missense mutations in the PTPN11 gene on MGCLS) has clinical similarities with Noonan’s syn- chromosome 12q24.1 have been identified as the cause of drome and cherubism. It is unclear whether it is a Noonan’s syndrome in 45% of familial and sporadic cases,45 distinct entity or a variant of Noonan’s syndrome or indicating genetic heterogeneity within the syndrome. In the cherubism. 5 study by Tartaglia et al, there was a family in which three N Three unrelated patients with NL/MGCLS were char- members had features of Noonan’s syndrome; two of these acterised, two of whom were found to have mutations had incidental mandibular giant cell lesions.3 All three in the PTPN11 gene, the mutation found in 45% of members were found to have a PTPN11 mutation known to patients with Noonan’s syndrome. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Utilization of Genomic Sequencing for Population Screening of Immunodeficiencies in the Newborn
© American College of Medical Genetics and Genomics ORIGINAL RESEARCH ARTICLE Utilization of genomic sequencing for population screening of immunodeficiencies in the newborn Ashleigh R. Pavey, MD1,2,3, Dale L. Bodian, PhD3, Thierry Vilboux, PhD3, Alina Khromykh, MD3, Natalie S. Hauser, MD3,4, Kathi Huddleston, PhD3, Elisabeth Klein, DNP3, Aaron Black, MS3, Megan S. Kane, PhD3, Ramaswamy K. Iyer, PhD3, John E. Niederhuber, MD3,5 and Benjamin D. Solomon, MD3,4,6 Purpose: Immunodeficiency screening has been added to many Results: WGS provides adequate coverage for most known state-directed newborn screening programs. The current metho- immunodeficiency-related genes. 13,476 distinct variants and dology is limited to screening for severe T-cell lymphopenia 8,502 distinct predicted protein-impacting variants were identified disorders. We evaluated the potential of genomic sequencing to in this cohort; five individuals carried potentially pathogenic augment current newborn screening for immunodeficiency, – variants requiring expert clinical correlation. One clinically including identification of non T cell disorders. asymptomatic individual was found genomically to have comple- Methods: We analyzed whole-genome sequencing (WGS) and ment component 9 deficiency. Of the symptomatic children, one clinical data from a cohort of 1,349 newborn–parent trios by was molecularly identified as having an immunodeficiency condi- genotype-first and phenotype-first approaches. For the genotype- tion and two were found to have other molecular diagnoses. first approach, we analyzed predicted protein-impacting variants in Conclusion: Neonatal genomic sequencing can potentially aug- 329 immunodeficiency-related genes in the WGS data. As a ment newborn screening for immunodeficiency. phenotype-first approach, electronic health records were used to identify children with clinical features suggestive of immunodefi- Genet Med advance online publication 15 June 2017 ciency.