Genetic Background of Meat Color and Its Impact on Meat Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chicken Catalog
Sugar Feather Farm Page: 1 Making Old Fashioned Fowl Great Again! TABLE OF CONTENTS Chicken ................................................................................................................... 2 Page: 1 Sugar Feather Farm Page: 2 Making Old Fashioned Fowl Great Again! CHICKEN Sugar Feather Farms has many specialty chickens for sale and we ship nationwide throughout the hatching season. You can mix and match your chicks in one order. We also offer Fully Feathered birds that are off heat and coop ready during season. These are aged 5 weeks to 9 weeks and these also can be sexed. Sugar Feather Farms takes pride in shipping healthy birds to satisfied customers. With so many different breeds of heritage or rare breeds, you may have trouble deciding which breed you want. To help with this decision, check out our descriptions for each breed to see who will match well with each other. This decision is based on several factors for your situation. Consultations for a small fee can be provided to get you off to a great start! All our chickens are treated with love, care and respect. We are a small farm who takes care of our fowl. We provide excellent nutrition for our birds all Non-GMO, this way they can be the healthiest possible. All chicks are vaccinated for Marek's Disease. If you do not want this vaccine please note this one the order. Page: 2 Sugar Feather Farm Page: 3 Making Old Fashioned Fowl Great Again! AMBERLINK CHICKEN HOW TO ORDER: Chicks: you can order now till October 13th - all chicks are sexed female and can be shipped or picked up. -

Annals of Warsaw University of Life Sciences – SGGW Annals of Warsaw University of Life
ISSN 1898-8830 Annals of Warsaw University of Life Sciences – SGGW Annals of Warsaw Annals of Warsaw University of Life Animal Science No 56 (2) 2017 Sciences – SGGW Animal Science No 56 (2) ISSN 1898-8830 1898 8830 2017 Agriculture (Agricultural and Forest Engineering) Animal Science Forestry and Wood Technology Horticulture and Landscape Architecture Land Reclamation Editorial Board Marian Binek Katarzyna Bogacka Bogdan Brzeziecki Bogdan Klepacki Włodzimierz Kluciński Anna Kołłajtis-Dołowy Andrzej Lenart Małgorzata Łobocka Józef Mosiej Arkadiusz Orłowski Małgorzata J. Riedel Marek S. Szyndel Jacek Wilkowski Janusz Wojdalski Michał Zasada WARSAW UNIVERSITY Distribution OF LIFE SCIENCES PRESS Annals are distributed by the Bookshop 166 Nowoursynowska St., 02-787 Warsaw of Warsaw University of Life Sciences Poland, tel. (48 22) 593 55 20 Press, 166 Nowoursynowska St., Warsaw, e-mail: [email protected] Poland. www.wydawnictwosggw.pl Agriculture (Agricultural and Forest Engineering) Animal Science Forestry and Wood Technology Horticulture and Landscape Architecture Land Reclamation Annals of Warsaw University of Life University of Life Sciences Press in two Sciences were originally published in 1957 copies. Papers submitted for consideration as Zeszyty Naukowe SGGW (Scienti- by the Editorial board should not exceed 0.5 fic Fascicles of SGGW). In 1980 the na- of a printed sheet (about 11 pages includ- me was changed to Annals of Warsaw ing illustrations, and should consist of the University of Life Sciences. following elements: 1) name and surname The Annals (5 subject series) are publi- of the author, 2) title of the paper, 3) abstract shed once or twice a year and will carry pre- (about 20 lines), 4) text of the paper, 5) date viously unpublished papers that are mainly in English, but also in French, German or when the paper was sent to the Warsaw Uni- Russian, followed by a short summary in versity of Life Sciences Press and mailing Polish. -
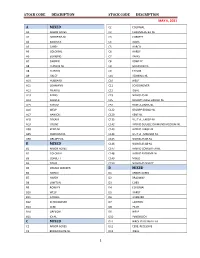
Stock Codes Numerical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C2 COLONIAL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 ANDREWS-NL C3 CORBETT A2 BABCOCK C4 DAVIS A3 CAREY C5 HARCO A5 COLONIAL C6 HARDY A6 EURIBRID C7 PARKS A7 GARBER C8 ROWLEY A8 H AND N-NL C9 GUILFORD-NL A8 H AND N C9 TATUM A9 HALEY C10 HENNING-NL A10 HUBBARD C10 WELP A11 LOHMANN C11 SCHOONOVER A12 MERRILL C12 IDEAL A13 PARKS C19 NICHOLAS-NL A14 SHAVER C35 ORLOPP LARGE BROAD-NL A15 TATUM C57 ROSE-A-LINDA-NL A16 WELP C122 ORLOPP BROAD-NL A17 HANSON C129 KENT-NL A18 DEKALB C135 B.U.T.A., LARGE-NL A19 HYLINE C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A38 KENT-NL C143 HYBRID LARGE-NL A45 MARCUM-NL C144 B.U.T.A., MEDIUM-NL A58 ORLOPP-NL C145 NICHOLAS 85-NL B MIXED C146 NICHOLAS 88-NL B1 ARBOR ACRES C147 HYBRID CONVERTER-NL B2 COLONIAL C148 HYBRID EXTREME-NL B3 CORBETT C149 MIXED B4 DAVIS C150 NICHOLAS SELECT B5 DEKALB WARREN D MIXED B6 HARCO D1 ARBOR ACRES B7 HARDY D2 BRADWAY B8 LAWTON D3 COBB B9 ROWLEY D4 COLONIAL B10 WELP D5 HARDY B11 CARGILL D6 HUBBARD B12 SCHOONOVER D7 LAWTON B13 CEBE D8 PILCH B14 OREGON D9 WELP B15 IDEAL D10 PENOBSCOT C MIXED D11 WROLSTAD SMALL-NL C1 ARBOR ACRES D11 CEBE, RECESSIVE C2 BROADWHITE-NL D12 IDEAL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N14 OLD ENGLISH, WHITE E MIXED N15 OLD ENGLISH, BLACK E1 COLONIAL N16 OLD ENGLISH, SPANGLED E2 HUBBARD N17 PIT E3 BOURBON, RED-NL N18 OLD ENGLISH E3 ROWLEY N19 MODERN E4 WELP N20 PIT, WHITE HACKLE E5 SCHOONOVER N21 SAM BIGHAM E6 CEBE N22 MCCLANHANS H MIXED N23 CLIPPERS H1 ARBOR ACRES N24 MINER BLUES H2 -

COMMUNITY CHICKEN PROJECT Koen Vanmechelen
COMMUNITY CHICKEN PROJECT Koen Vanmechelen Open University of Difference (OpUnDi), Wasserman Projects, Detroit (US), 2016 CCP, The Future of Hope, Harare (ZW), 2015 CCP, De Hoev, Zonhoven (BE), 2015 La Biomista, Genk (BE), 2015-09-22 Artist Koen Vanmechelen COMMUNITY CHICKEN PROJECT Koen Vanmechelen Modern poultry flocks have become genetically impoverished due to industrialised farming and are at considerable risk from diseases. The continuous crossbreeding of chicken species in the Cosmopolitan Chicken Project (CCP), which is artistic-philosophical in nature has resulted in the birth of chickens that show a remarkably higher genetic diversity. Their immense gene pool consists of a whopping 13 million single-nucleotide polymorphisms (SNPs). The Cosmopolitan Chicken Research Project (CC®P) studies them and revealed the genetic enrichment through crossbreeding. A new project, the Community Chicken Project has to prevent inbreeding by injecting the genetic diversity into other species. The layout of the plan is like this: every year a rooster of the newest crossbreed will be paired with a regular egg laying chicken. This chicken will absorb the genetic pool of the CCP rooster and produce a vital community chicken who can provide the communities it lives in with healthy eggs. The time is now. The growing popularity of chickens is a fact, but at the same time the food insecurity increases. Even in richer countries the sudden halt of food supplies through calamities, wars, riots or climate change can lead to much misery. The need for food security and food independency of traditional industrial supply has played a pivotal role in the growing resurgence of small-scale agriculture, from raising homegrown vegetables to homebred animals. -

Backyard February/March 2016
Volume 11, Number 1 Backyard February/March 2016 PoultryDedicated to more and better small-flock poultry Antioxidants to the Rescue How the right diet can help your flock live longer and healthier An Introduction to Raising Geese A Profile of the Cream Legbar Breed Debunking Chicken Myths $4.99 US www.backyardpoultrymag.com Backyard Poultry FP 12-15 THINK:Mother Earth 4.5 x7 12/17/15 3:20 PM Page 1 SATISFACTION GUARANTEED oryourmoneyback! To Protect Your Property From Night Predator Animals Nite•Guard Solar® has been proven effective in repelling predator animals for the past 19 years. #1 Nite•Guard Solar attacks the deepest most primal The World’s fear of night animals, that of being discovered. Top Selling Solar Powered Nite•Guard When the sun goes down, Nite•Guard begins to Security System Repellent Tape DON’T BE FOOLED BY Keeps predators flash and continues until sunrise. The simple COPY CATS away during the but effective fact is that a “flash of light” is daylight hours sensed as an eye and becomes an immediate 95 $14 Per Roll threat to the most ferocious night animals and they will run away. PO Box 274 • Princeton MN 55371 • 1.800.328.6647 ......................... For information and videos, see us at FAMILY OWNED AND OPERATED SINCE 1997 www.niteguard.com ......................... NO NOEGG EGG EGG NO DISCARDDISCARD DISCARD ALL NATURAL NATURAL ALL FORMULATEDALL NATURAL FOR CHICKENS, TURKEYS, TURKEYS, CHICKENS, FOR FORMULATED DUCKS, GEESE, DOMESTIC POULTRY, POULTRY, DOMESTIC GEESE, DUCKS, ANDFORMULATED WATERFOWL FOR OF ALLCHICKENS, -

Performance and Meat Quality of Dual-Purpose Cockerels of Dominant Genotype Reared on Pasture
animals Article Performance and Meat Quality of Dual-Purpose Cockerels of Dominant Genotype Reared on Pasture Michaela Englmaierová * , Miloš Skˇrivan,Tomáš Taubner and VˇeraSkˇrivanová Department of Nutrition Physiology and Animal Product Quality, Institute of Animal Science, 104 00 Prague-Uhrineves, Czech Republic; [email protected] (M.S.); [email protected] (T.T.); [email protected] (V.S.) * Correspondence: [email protected]; Tel.: +420-267-009-547 Received: 31 January 2020; Accepted: 24 February 2020; Published: 27 February 2020 Simple Summary: One-day-old laying cockerels are killed after hatching because they do not reach the growth rate of broiler chickens, and their fattening would be economically disadvantageous. A possible variant of the use of these cockerels could be organic or free-range farming, where it is desirable that the animals are fattened for a longer period of time, are more physically active, and graze pasture vegetation. Another possibility is dual-purpose genotype breeding, where hens are used for egg production and cockerels are fattened for meat. In the present study, three genotypes of dual-purpose cockerels Dominant were compared. All three genotypes showed the ability to graze with resulting improvements in meat quality. The Dominant 102 cockerels have the greatest prerequisites for use in extensive fattening, mainly due to higher performance, willingness to graze and vitamin E content, which protects the fat from becoming rancid. Abstract: The culling of layer cockerels due to economic inefficiency is an ethical problem. Organic or free-range fattening of these cockerels or dual-purpose genotypes breeding is a possible solution to this problem. -

Fibromelanosis in Domestic Chickens.Cdr
ISSN 1313 - 8820 Volume 5, Number 3 September 2013 2013 Editor-in-Chief Scope and policy of the journal for rewriting to the authors, if necessary. Agricultural Science and Technology /AST/ The editorial board reserves rights to reject Tsanko Yablanski – an International Scientific Journal of manuscripts based on priorities and space Faculty of Agriculture Agricultural and Technology Sciences is availability in the journal. Trakia University, Stara Zagora published in English in one volume of 4 Bulgaria issues per year, as a printed journal and in The articles appearing in this journal are electronic form. The policy of the journal is indexed and abstracted in: EBSCO to publish original papers, reviews and Publishing, Inc. and AGRIS (FAO). Co-Editor-in- Chief short communications covering the The journal is accepted to be indexed with aspects of agriculture related with life the support of a project № BG051PO001- Radoslav Slavov sciences and modern technologies. It will 3.3.05-0001 “Science and business” Faculty of Agriculture offer opportunities to address the global financed by Operational Programme Trakia University, Stara Zagora needs relating to food and environment, “Human Resources Development” of EU. Bulgaria health, exploit the technology to provide The title has been suggested to be innovative products and sustainable included in SCOPUS (Elsevier) and development. Papers will be considered in Electronic Journals Submission Form Editors and Sections aspects of both fundamental and applied (Thomson Reuters). science in the areas of Genetics and Genetics and Breading Breeding, Nutrition and Physiology, Internet Access Production Systems, Agriculture and This journal is included in the Trakia Atanas Atanasov (Bulgaria) Environment and Product Quality and University Journals online Service which Ihsan Soysal (Turkey) Safety. -

The Cosmopolitan Chicken Project (Ccp) 1999 — 2016
THE COSMOPOLITAN CHICKEN PROJECT (CCP) 1999 — 2016 × × × ♂ MECHELSE KOEKOEK POULET DE BRESSE ♀ ♀ MECHELSE LOUISIANA ♂ ♂ MECHELSE ♀ Belgium France CCP Thailand CCP China × × × ♀ MECHELSE BRESSE ENGLISH REDCAP ♂ ♂ MECHELSE AURACANA ♀ ♂ MECHELSE ♀ CCP England CCP Brazil CCP Egypt × × × ♀ JERSEY GIANT MECHELSE REDCAP ♂ ♀ DENIZLI HOROZU MECHELSE AURACANA ♂ ♀ POULET DE SENEGAL MECHELSE ♂ U.S.A. CCP Turkey CCP Senegal CCP × ♂ MECHELSE CEMANI WYANDOTTE ♀ CCP Detroit × × × ♂ MECHELSE GIANT DRESDNER ♀ ♀ MECHELSE DENIZLI CUBALAYA ♂ ♀ MECHELSE SENEGAL STYRIAN ♂ CCP Germany CCP Cuba CCP Slovenia × × × ♀ UILEBAARD MECHELSE DRESDNER ♂ ♀ ANCONA MECHELSE CUBALAYA ♂ ♂ SULMTALER MECHELSE STYRIAN ♀ The Netherlands CCP Italy CCP Austria CCP × × × ♂ MECHELSE UILEBAARD LOUISIANA ♀ ♂ MECHELSE ANCONA ♀ ♀ MECHELSE SULMTALER AYAM CEMANI ♂ CCP Mexico CCP Russia CCP Indonesia THE COSMOPOLITAN CHICKEN PROJECT (CCP) DIVERSITY AND DUALISM Nothing is what it seems. That is but one of the many generation, new blood is needed. “Every organism The Cosmopolitan Chicken Project: approach - much more surprising - lessons of the Cosmopolitan needs some other organism in order to survive,” says and state of progress Chicken Project (CCP), a worldwide crossbreeding Vanmechelen, “If there is one secret in life, maybe it’s program involving national and regional chicken that everything is dual. Chicken and egg, cause and The first generation hybrid, the Mechelse Bresse, breeds that started in 1998 by visual artist Koen Van- effect, good and bad, construction and destruction: resulted from crossbreeding the Belgian Mechelse mechelen (b. 1965, Sint-Truiden, Belgium). everything exists in relation to the Other. If you pursue Koekoek - the pride of Flemish chicken farmers - this line of reasoning, you might even conclude that with the top French chicken, the Poulet de Bresse. -

Animal Genetic Resources
20786336_51-0.qxd 12/17/12 6:40 PM Page 1 51 2012 51 ANIMAL GENETIC RESOURCES • RESSOURCES GÉNÉTIQUES ANIMALES • RECURSOS GENÉTICOS ANIMALES 2012 ANIMAL GENETIC ISSN 2078-6336 RESOURCES an international journal RESSOURCES GÉNÉTIQUES ANIMALES un journal international RECURSOS GENÉTICOS ANIMALES una revista internacional FAO ANIMAL GENETIC Editor-in-Chief B. Scherf RESOURCES Editors S. Galal; I. Hoffmann 51 ESSOURCES Viale delle Terme di Caracalla, 00153 Rome, R Italy GÉNÉTIQUES ANIMALES Animal Genetic Resources is an international journal 2012 published under the auspices of the Animal Genetic RECURSOS Resources Branch of the Animal Production and Health Division, Food and Agriculture Organization GENÉTICOS ANIMALES of the United Nations (FAO). Ressources génétiques animales est un journal international publié sous les auspices de la Sous- Division des ressources génétiques animales de la CONTENTS Page Division de la production et de la santé animales, Organisation des Nations Unies pour l’alimentation Editorial .......................................................................................................................... iii ’ et l agriculture (FAO). Fipa cattle in the southwestern highlands of Tanzania: socio-economic Recursos genéticos animales es una revista inter- roles, traditional management practices and production constraints nacional publicada bajo los auspicios de la P.L. Mwambene, A.M. Katule, S.W. Chenyambuga & P.A.A. Mwakilembe ....................1 Subdivisión de los Recursos Genéticos Animales de la División de Producción y Sanidad Animal, Fipa cattle in the southwestern highlands of Tanzania: morphometric la Organización de las Naciones Unidas para la and physical characteristics Alimentación y la Agricultura (FAO). P.L. Mwambene, A.M. Katule, S.W. Chenyambuga & P.A.A. Mwakilembe ..................15 Fipa cattle in the southwestern highlands of Tanzania: molecular Print edition and institutional subscriptions / characterization Édition imprimée et abonnements pour insti- P.L. -

Kort Allerlei
VARIOUSVARIOUS Part 1a October 2013 For next or previous screen: use the mouse wheel, or Pg Up and Pg Dn or ↑ and ↓ on your keyboard. A word from the editor……. Dear reader, A nice and thick autumn issue is ready for you to read. You see, we do not cut corners on the pages! Yet it is every time a considerable challenge to get it all online for you. And also in two languages! The name of the author and the photographer is always stated in an article, but there are also a number of 'silent workers' who help translate, and so-called 'native speakers' who then apply the finishing touch. Each in his profession, so the pigeons, poultry and waterfowl articles go to an expert in that field. We like to put these people in the spotlight by naming them here: In the Netherlands: Luuk Hans, Henk Meijers and Willem van Ballekom. In Germany: Mick Bassett, Thomas Hellmann and Arjan Warnshuis. In England: Denise Moss, Rinke Berkenbosch, Jim Mullan, Michael Hart and Frank Bridgland. In Australia: Greg Davies and Erik Berrevoets. In Romania: Bobo Athes and in de USA: Bill Burnside. Without their help Aviculture Europe could not exist! Moreover, there are always people welcome who occasionally want to help translate Dutch into English or vice versa, many hands make light work! Please contact the chief editor at [email protected] if you want to join the team. We wish you pleasant reading and for those who have not done it already: please subscribe to our newsletter by sending us your e-mail address, so that you will be automatically informed about the publishing of each new issue. -

List of Chicken Breeds by Place of Origin
https://en.wikipedia.org/wiki/List_of_chicken_breeds List of chicken breeds by place of origin: Afghanistan Black Shumen France Ingriido Starozagorska Red Crèvecœur Khasaki La Flèche Kulangi Canada Faverolles Pusty Chantecler Aquitaine Sabzwari Red Shaver Ardeale Ardennaise, or Sans-queue des Ardennes Albania Chile Barbezieux Commune hen Araucana Blanzac Black Tropoja Lekbibaj Bourbonnaise Tirana China Bourbourg Tropoja Pac A Silkie hen Bresse Gauloise Yerevanian Cochin Caumont Croad Langshan Caussade Australia Nankin Charollaise Australorp Pekin Cocherelle Australian Langshan Silkie Combattant du Nord Australian Game Contres Australian Pit Game Croatia Cotentine Hrvatica Coucou des Flandres Austria Coucou de France Altsteirer Cuba Coucou de Rennes Sulmtaler Cubalaya Coucou Picarde Coucou Soie Belgium Cyprus Cou-nu du Forez The d'Everberg Cypriot chicken Courtes-Pattes Ardennaise or Ardenner Crèvecœur Ardenner Bolstaart Czech Republic Estaires Belgian Bearded d'Anvers, Antwerp Czech gold brindle chicken Faverolles clair (light) Belgian Faverolles foncée (dark or German Barbu de Boitsfort or Bosvoordse Egypt Faverolles) baardkriel Alexandria Favoris Barbu de Grubbe or Grubbe baardkriel Arbrikers Gasconne Belgian d'Everberg, Barbu d'Everberg Bahig Gâtinaise Barbu de Watermael or Watermaalse Bandara Gauloise dorée baardkriel Boffen Géline de Touraine Bearded d'Uccle, Barbu d'Uccle or Ukkelse Dandarawi Gournay baardkriel Dokki 4 Hergnies Bassette Liègeoise Baladi Beheri Houdan Brabanter, Brabançonne or Brabants hoen Fayoumi Ivanaise Braekel -

OBAT HEWAN DI INDONESIA Subdit Pengawasan Obat Hewan Ditkeswan
Disampaikan Pada Seminar Bisnis Peternakan 22 November 2018 drh. Fadjar Sumping Tjatur Rasa, PhD. DIREKTUR KESEHATAN HEWAN UU No. 18/2009 • Pasal 50 • Obat hewan yang dibuat dan disediakan dgn maksud untuk diedarkan harus memiliki NOMOR PENDAFTARAN. • Pembuatan, penyediaan, peredaran dan pengujian obat hewan harus dilakukan di bawah pengawasan OTORITAS VETERINER. • 4)Pemerintah dan Pemerintah Daerah sesuai dengan kewenangannya melakukan pengawasan atas pembuatan, penyediaan, dan peredaran obat hewan. 2 Pasal 52 Ayat (1) Setiap orang yang berusaha di bidang pembuatan, penyediaan, dan/atau peredaran obat hewan wajib memiliki izin usaha sesuai dengan ketentuan peraturan perundang-undangan Ayat (2) Setiap orang DILARANG membuat, menyediakan, dan/atau mengedarkan obat hewan yang 1. berupa sediaan biologik yang penyakitnya tidak ada di Indonesia; 2. tidak memiliki nomor pendaftaran; 3. tidak diberi label dan tanda; dan 4. tidak memenuhi standar mutu 3 Pasal 54 ayat (1) Penyediaan obat hewan dilakukan dengan mengutamakan produksi dalam negeri Pasal 54 ayat (4) Pengeluaran obat hewan produksi dalam negeri ke luar negeri harus mengutamakan kepentingan nasional 4 Permentan No. 18/2009 • Usaha obat hewan meliputi pembuatan/produksi, penyediaan, peredaran obat hewan, pemasukan dan/atau pengeluaran obat hewan DARI DAN KE LUAR NEGERI. • Usaha obat hewan dapat dilakukan oleh perorangan warga negara Indonesia atau badan usaha. Subdirektorat Pengawasan Obat Hewan 5 PENGGOLONGAN IZIN USAHA OBAT HEWAN PERSYARATAN PENERBITAN IZIN USAHA • Pabrik, sarana dan