The Mechanism of the Origination of Auto-Allopolyploidy and Aneuploidy in Higher Plants Based on the Cases of Iris and Triticeae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Karyological Studies on Iris Japonica Thunb. and Its Allies1) 2) by K
180 Cytologia 10 Karyological Studies on Iris japonica Thunb. and Its Allies1) 2) By K. Yasui Tokyo Imperial University (With 8 text figures) Receic ed MTV 28, 1939 Introduction There are several karyological investigations on Iris japonica. KAZAO (1928) considered the sporophyte of this species as an auto triploid having 54 chromosomes. But SIMONET (1932, 1934) re ported I. japonica as a diploid plant with 34 chromosomes in its root-tip cells, and considered I. japonica var aphrodite as a hyper triploid and interpreted the chromosome constitution as 2n=51+3, the latter 3 chromosomes being considered as derived from 3 of 51 chromosomes in I. japonica by fragmentation which occurred att their median constrictions. The karyological investigation in I. japonica and the other 2 allied species, which were kindly put at my deposal by Dr. S. IMA MURA of Kyoto Imp. University, gave me some results different from those of the previous authors as is described below. Material and Method In the fixing of the root-tip cells both NAVASHIN's and LEWITSKY's solutions were used. When fixed in the latter, the chromosomes were found longer and slender than when fixed in the former solution, but there was no essential difference in the distribu tion of chromosomes in the equatorial plate. NEWTON'S gentian violet staining was generally adopted. Materials for the study of meiosis were fixed mostly with NAVASHIN'S fluid and stained with HEIDENHAIN'S iron-alum haematoxylin. Acetocarmine smears were also tried in the study of the pollen mother cells. Drawings were made with a camera lucida. -
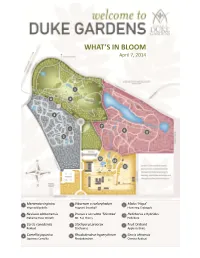
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Four New Early Spring-Flowering Evergreen Iris Cultivars
CULTIVAR AND GERMPLASM RELEASES HORTSCIENCE 55(1):103–105. 2020. https://doi.org/10.21273/HORTSCI14433-19 Dawn’ (‘Xiaodie’), ‘Butterflies in Bloom’ (‘Huadie’), and ‘Butterfly Veil’ (‘Dieyi’) by Four New Early Spring-flowering the American Iris Society in 2019. Evergreen Iris Cultivars Description Feng-yang Yu and Yue-e Xiao The selection process was conducted at the Conservation Nursery of the Shanghai Research Center, Shanghai Botanical Garden, Shanghai, 200231, China; Botanical Garden in Shanghai, China. For and Shanghai Engineering Research Center of Sustainable Plant Innovation, each of the four new cultivars, 30 clones were Shanghai 200231, China planted (4 cultivars · 30 clones) in Sept. 2016. The plants were grown in arrays with Lin Cheng 30 cm between plants in the experimental College of Biology and Environment, Nanjing Forest University, Nanjing field; they were irrigated and fertilized simi- 210037, China larly to other perennial herbs. All plants were grown at a forest edge where they received half Shu-cheng Feng and Lei-lei Zhang full-sunlight. Morphological characteristics in- Research Center, Shanghai Botanical Garden, Shanghai, 200231, China; cluding plant height, leaf length and width, and Shanghai Engineering Research Center of Sustainable Plant Innovation, flower color, flower diameter, inner perianth length and width, outer perianth length and Shanghai 200231, China width, and the flowering period of the total Additional index words. cultivar, flower period, Iris japonica, ornamental, selection population. Single flowers were evaluated for a randomized sample of 15 plants per cultivar. Leaf length and width were measured on the Iris, with its showy and colorful flowers, is from late March to mid-April in Shanghai third leaf from the top of each plant. -

Pollen and Stamen Mimicry: the Alpine Flora As a Case Study
Arthropod-Plant Interactions DOI 10.1007/s11829-017-9525-5 ORIGINAL PAPER Pollen and stamen mimicry: the alpine flora as a case study 1 1 1 1 Klaus Lunau • Sabine Konzmann • Lena Winter • Vanessa Kamphausen • Zong-Xin Ren2 Received: 1 June 2016 / Accepted: 6 April 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Many melittophilous flowers display yellow and Dichogamous and diclinous species display pollen- and UV-absorbing floral guides that resemble the most com- stamen-imitating structures more often than non-dichoga- mon colour of pollen and anthers. The yellow coloured mous and non-diclinous species, respectively. The visual anthers and pollen and the similarly coloured flower guides similarity between the androecium and other floral organs are described as key features of a pollen and stamen is attributed to mimicry, i.e. deception caused by the flower mimicry system. In this study, we investigated the entire visitor’s inability to discriminate between model and angiosperm flora of the Alps with regard to visually dis- mimic, sensory exploitation, and signal standardisation played pollen and floral guides. All species were checked among floral morphs, flowering phases, and co-flowering for the presence of pollen- and stamen-imitating structures species. We critically discuss deviant pollen and stamen using colour photographs. Most flowering plants of the mimicry concepts and evaluate the frequent evolution of Alps display yellow pollen and at least 28% of the species pollen-imitating structures in view of the conflicting use of display pollen- or stamen-imitating structures. The most pollen for pollination in flowering plants and provision of frequent types of pollen and stamen imitations were pollen for offspring in bees. -

Sistemática Y Evolución De Encyclia Hook
·>- POSGRADO EN CIENCIAS ~ BIOLÓGICAS CICY ) Centro de Investigación Científica de Yucatán, A.C. Posgrado en Ciencias Biológicas SISTEMÁTICA Y EVOLUCIÓN DE ENCYCLIA HOOK. (ORCHIDACEAE: LAELIINAE), CON ÉNFASIS EN MEGAMÉXICO 111 Tesis que presenta CARLOS LUIS LEOPARDI VERDE En opción al título de DOCTOR EN CIENCIAS (Ciencias Biológicas: Opción Recursos Naturales) Mérida, Yucatán, México Abril 2014 ( 1 CENTRO DE INVESTIGACIÓN CIENTÍFICA DE YUCATÁN, A.C. POSGRADO EN CIENCIAS BIOLÓGICAS OSCJRA )0 f CENCIAS RECONOCIMIENTO S( JIOI ÚGIC A'- CICY Por medio de la presente, hago constar que el trabajo de tesis titulado "Sistemática y evo lución de Encyclia Hook. (Orchidaceae, Laeliinae), con énfasis en Megaméxico 111" fue realizado en los laboratorios de la Unidad de Recursos Naturales del Centro de Investiga ción Científica de Yucatán , A.C. bajo la dirección de los Drs. Germán Carnevali y Gustavo A. Romero, dentro de la opción Recursos Naturales, perteneciente al Programa de Pos grado en Ciencias Biológicas de este Centro. Atentamente, Coordinador de Docencia Centro de Investigación Científica de Yucatán, A.C. Mérida, Yucatán, México; a 26 de marzo de 2014 DECLARACIÓN DE PROPIEDAD Declaro que la información contenida en la sección de Materiales y Métodos Experimentales, los Resultados y Discusión de este documento, proviene de las actividades de experimen tación realizadas durante el período que se me asignó para desarrollar mi trabajo de tesis, en las Unidades y Laboratorios del Centro de Investigación Científica de Yucatán, A.C., y que a razón de lo anterior y en contraprestación de los servicios educativos o de apoyo que me fueron brindados, dicha información, en términos de la Ley Federal del Derecho de Autor y la Ley de la Propiedad Industrial, le pertenece patrimonialmente a dicho Centro de Investigación. -

Iris Sibirica and Others Iris Albicans Known As Cemetery
Iris Sibirica and others Iris Albicans Known as Cemetery Iris as is planted on Muslim cemeteries. Two different species use this name; the commoner is just a white form of Iris germanica, widespread in the Mediterranean. This is widely available in the horticultural trade under the name of albicans, but it is not true to name. True Iris albicans which we are offering here occurs only in Arabia and Yemen. It is some 60cm tall, with greyish leaves and one to three, strongly and sweetly scented, 9cm flowers. The petals are pure, bone- white. The bracts are pale green. (The commoner interloper is found across the Mediterranean basin and is not entitled to the name, which continues in use however. The wrongly named albicans, has brown, papery bracts, and off-white flowers). Our stock was first found near Sana’a, Yemen and is thriving here, outside, in a sunny, raised bed. Iris Sibirica and others Iris chrysographes Black Form Clumps of narrow, iris-like foliage. Tall sprays of darkest violet to almost black velvety flowers, Jun-Sept. Ht 40cm. Moist, well drained soil. Part shade. Deepest Purple which is virtually indistinguishable from black. Moist soil. Ht. 50cm Iris chrysographes Dykes (William Rickatson Dykes, 1911, China); Section Limniris, Series Sibericae; 14-18" (35-45 cm), B7D; Flowers dark reddish violet with gold streaks in the signal area giving it its name (golden writing); Collected by E. H. Wilson in 1908, in China; The Gardeners' Chronicle 49: 362. 1911. The Curtis's Botanical Magazine. tab. 8433 in 1912, gives the following information along with the color illustration. -

Ulster Group Newsletter 2013.Pdf
Newsletter No:12 Contents:- Editorial Obituaries Contributions:- Notes on Lilies Margaret and Henry Taylor Some Iris Species David Ledsham 2nd Czech International Rock Garden Conference Kay McDowell Homage to Catalonia Liam McCaughey Alpine Cuttings - or News Items Show News:- Information:- Web and 'Plant of the Month' Programme 2013 -2014 Editorial After a long cold spring I hope that all our members have been enjoying the beautiful summer, our hottest July for over 100 years. In the garden, flowers, butterflies and bees are revelling in the sunshine and the house martins, nesting in our eaves, are giving flying displays that surpass those of the Red Arrows. There is an emphasis ( almost a fashion) in horticultural circles at the moment on wild life gardening and wild flower meadows. I have always felt that alpines are the wild flowers of the mountains, whether growing in alpine meadows or nestling in among the rocks. Our Society aims to give an appreciation and thus the protection and conservation of wild flowers and plants all over the world. Perhaps you have just picked up this Newsletter and are new to the Society but whether you have a window pot or a few acres you would be very welcome to join the group and find out how much pleasure, in many different ways, these mountain wild flowers can bring. My thanks to our contributors this year who illustrate how varied our interest in plants can be. Not only did the Taylors give us a wonderful lecture and hands-on demonstration last November but kindly followed it up with an article for the Newsletter, and I hope that many of you, like me, have two healthy little pots of lily seedlings thanks to their generous gift of seeds. -

Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes
Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes Shichao Chen1., Dong-Kap Kim2., Mark W. Chase3, Joo-Hwan Kim4* 1 College of Life Science and Technology, Tongji University, Shanghai, China, 2 Division of Forest Resource Conservation, Korea National Arboretum, Pocheon, Gyeonggi- do, Korea, 3 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, United Kingdom, 4 Department of Life Science, Gachon University, Seongnam, Gyeonggi-do, Korea Abstract Phylogenetic analysis aims to produce a bifurcating tree, which disregards conflicting signals and displays only those that are present in a large proportion of the data. However, any character (or tree) conflict in a dataset allows the exploration of support for various evolutionary hypotheses. Although data-display network approaches exist, biologists cannot easily and routinely use them to compute rooted phylogenetic networks on real datasets containing hundreds of taxa. Here, we constructed an original neighbour-net for a large dataset of Asparagales to highlight the aspects of the resulting network that will be important for interpreting phylogeny. The analyses were largely conducted with new data collected for the same loci as in previous studies, but from different species accessions and greater sampling in many cases than in published analyses. The network tree summarised the majority data pattern in the characters of plastid sequences before tree building, which largely confirmed the currently recognised phylogenetic relationships. Most conflicting signals are at the base of each group along the Asparagales backbone, which helps us to establish the expectancy and advance our understanding of some difficult taxa relationships and their phylogeny. -

Plant Guide Spring
Plant Guide Spring China is home to more than 30,000 plant species – one-eighth of the world’s total. At Lan Su, visitors can enjoy hundreds of these plants, many of which have a rich symbolic and cultural history in China. This guide is a selected look at some of Lan Su’s current favorites. Please return this guide to the Garden Host at the entrance when your visit is over. A Clematis G Magnolia* M Rhododendron* B Chinese Paper Bush H Lushan Honeysuckle N Winter Jasmine C Winter Daphne I Peony* O Kerria D Chinese Fringe Flower J Chinese Plum P Bergenia E Forsythia K Quince Q Iris F Camelllia* L Crabapple R Corydalis * For a complete list of these species, please request a master species list at the entrance. It is also available online at www.lansugarden.org/plants PLANT Guide Spring Clematis A Forsythia E (Clematis armandii ‘Appleblossom’, (Forsythia x intermedia ‘Lynwood Gold’) C. fasciculiflora) Long cultivated in Chinese gardens, C. armandii is native to China. Look forsythia has become popular in for the soft-pink, lightly fragrant gardens throughout the world. Cut ‘Apple Blossom’ cultivar above the branches can be forced to bloom early, moon gate. C. fasciculiflora is a rare when brought indoors. Chinese species with small, fragrant white flowers. Its young foliage has distinctive, silver center stripes. Chinese Paper B Camellia F Bush (Camellia. japonica ‘Drama Girl’) For additional camellia varieties, see the Master (Edgeworthia ‘Red Dragon’, E. chrysantha) Species List Native to China, this deciduous shrub is a relative of sweet Daphne. -

Phytophoto Index 2018
PhytoPhoto 2018 Image Availability Accessing the photo collection is easy. Simply send an email with the plant names or a description of images sought to [email protected] and a gallery of photos meeting your criteria will be submitted to you, usually the same day. Abeliophyllum distichum Abutilon vitifolium ‘Album’ Acer palmatum fall color Abeliophyllum distichum ‘Roseum’ Abutilon vitifolium white Acer palmatum in front of window Abelmoschus esculentus "Okra" Abutilon Wisley Red Acer palmatum in orange fall color Abelmoschus manihot Abutilon x hybridum 'Bella Red' Acer palmatum var. dissectum Abies balsamea 'Nana' Abutilon-orange Acer palmatum var. dissectum Dissectum Abies concolor 'Blue Cloak' Abutilon-white Viride Group Abies guatemalensis Acacia baileyana Acer pensylvaticum Abies koreana 'Glauca' Acacia baileyana 'Purpurea' Acer platanoides 'Princeton Gold' Abies koreana 'Green Carpet' Acacia boormanii Acer pseudoplatanus Abies koreana 'Horstmann's Silberlocke' Acacia confusa Acer pseudoplatanus 'Leopoldii' Abies koreana 'Silberperle' Acacia cultriformis Acer pseudoplatanus 'Purpureum' Abies koreana 'Silberzwerg' Acacia dealbata Acer pseudoplatanus ‘Puget Pink’ Abies koreana 'Silver Show' Acacia iteaphylla Acer pseudoplatanus f... 'Leopoldii' Abies koreana Aurea Acacia koa Acer rubrum Abies koreana-cone Acacia koa seedlings Acer rubrum and stop sign Abies lasiocarpa Acacia koaia Acer rufinerve Hatsuyuki Abies lasiocarpa v. arizonica 'Argentea' Acacia longifolia Acer saccharinum Abies lasiocarpa v. arizonica 'Glauca Acacia -

Secondary Metabolites of the Choosen Genus Iris Species
ACTA UNIVERSITATIS AGRICULTURAE ET SILVICULTURAE MENDELIANAE BRUNENSIS Volume LX 32 Number 8, 2012 SECONDARY METABOLITES OF THE CHOOSEN GENUS IRIS SPECIES P. Kaššák Received: September 13,2012 Abstract KAŠŠÁK, P.: Secondary metabolites of the choosen genus iris species. Acta univ. agric. et silvic. Mendel. Brun., 2012, LX, No. 8, pp. 269–280 Genus Iris contains more than 260 species which are mostly distributed across the North Hemisphere. Irises are mainly used as the ornamental plants, due to their colourful fl owers, or in the perfume industry, due to their violet like fragrance, but lot of iris species were also used in many part of the worlds as medicinal plants for healing of a wide spectre of diseases. Nowadays the botanical and biochemical research bring new knowledge about chemical compounds in roots, leaves and fl owers of the iris species, about their chemical content and possible medicinal usage. Due to this researches are Irises plants rich in content of the secondary metabolites. The most common secondary metabolites are fl avonoids and isofl avonoids. The second most common group of secondary metabolites are fl avones, quinones and xanthones. This review brings together results of the iris research in last few decades, putting together the information about the secondary metabolites research and chemical content of iris plants. Some clinical studies show positive results in usage of the chemical compounds obtained from various iris species in the treatment of cancer, or against the bacterial and viral infections. genus iris, secondary metabolites, fl avonoids, isofl avonoids, fl avones, medicinal plants, chemical compounds The genus Iris L.