Secondary Metabolites of the Choosen Genus Iris Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Weed Risk Assessment for Iris Pseudacorus L. (Iridaceae)
Weed Risk Assessment for Iris United States pseudacorus L. (Iridaceae) – Yellow Department of flag iris Agriculture Animal and Plant Health Inspection Service September 24, 2013 Version 1 Left: Iris pseudacorus flower. Right: A colony of Iris pseudacorus (source: Bugwood, 2013). Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology Plant Protection and Quarantine Animal and Plant Health Inspection Service United States Department of Agriculture 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Weed Risk Assessment for Iris pseudacorus Introduction Plant Protection and Quarantine (PPQ) regulates noxious weeds under the authority of the Plant Protection Act (7 U.S.C. § 7701-7786, 2000) and the Federal Seed Act (7 U.S.C. § 1581-1610, 1939). A noxious weed is defined as “any plant or plant product that can directly or indirectly injure or cause damage to crops (including nursery stock or plant products), livestock, poultry, or other interests of agriculture, irrigation, navigation, the natural resources of the United States, the public health, or the environment” (7 U.S.C. § 7701-7786, 2000). We use weed risk assessment (WRA)— specifically, the PPQ WRA model (Koop et al., 2012)—to evaluate the risk potential of plants, including those newly detected in the United States, those proposed for import, and those emerging as weeds elsewhere in the world. Because the PPQ WRA model is geographically and climatically neutral, it can be used to evaluate the baseline invasive/weed potential of any plant species for the entire United States or for any area within it. As part of this analysis, we use a stochastic simulation to evaluate how much the uncertainty associated with the analysis affects the model outcomes. -

Morphological Peculiarities of Fruits of the Rare Species Iris Halophila Pall, I. Pumila L. and I. Hungarica Waldst. Et Kit
Plant Introduction, 85/86, 85–92 (2020) https://doi.org/10.46341/PI2020007 UDC 581.47:582.579.2(477-25) RESEARCH ARTICLE Morphological peculiarities of fruits of the rare species Iris halophila Pall, I. pumila L. and I. hungarica Waldst. et Kit. (Iridaceae Juss.) in the conditions of introduction in the meadow-steppe cultural phytocenosis V.V. Gritsenko M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, Timiryazevska str. 1, 01014 Kyiv, Ukraine; [email protected] Received: 27.01.2020 | Accepted: 23.05.2020 | Published: 30.06.2020 Abstract The objective of this study was to analyze the morphological structure and to reveal common and distinguishing features of the fruit in rare steppe species Iris halophila, I. pumila and I. hungarica introduced in conditions of meadow-steppe cultural phytocenosis in the M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine (NBG). Material and methods. Fruits of I. halophila, I. pumila and I. hungarica were collected on the botanical- geographical plot “Steppes of Ukraine” of NBG during 2015–2019. Fruit parameters were measured using a regular ruler. Morphological terms are provided, according to Artyushenko & Fedorov (1986). Colors were determined by Bondartsev’s (1954) scale. Results. In all analyzed species, the fruit is a trimeric and trilocular loculicidal capsule with multi-seeded locules. This capsule is erect, straight, leathery, glabrous, opening by dehiscence from top to bottom along the dorsal veins of carpels. The morphological peculiarities of the fruits, which may be additional diagnostic characters of these species, are established. In particular, in I. halophila capsule is cylindrical, with the upper part elongated into the apical spout (long, thin, bent to the side). -

The Mongolian Local Knowledge on Plants Recorded in Mongolia and Amdo and the Dead City of Khara-Khoto and Its Scienti�C Value
The Mongolian Local Knowledge on Plants Recorded in Mongolia and Amdo and the Dead City of Khara-Khoto and Its Scientic Value Guixi Liu ( [email protected] ) IMNU: Inner Mongolia Normal University https://orcid.org/0000-0003-3354-2714 Wurheng Wurheng IMNU: Inner Mongolia Normal University Khasbagan Khasbagan IMNU: Inner Mongolia Normal University Yanying Zhang IMNU: Inner Mongolia Normal University Shirong Guo IMNU: Inner Mongolia Normal University Research Keywords: P. K. Kozlov, Expedition Record, Local Knowledge on plants, Mongolian Folk, Ethnobotany, Botanical History Posted Date: December 28th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-133605/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License The Mongolian local knowledge on plants recorded in Mongolia and Amdo and the Dead City of Khara-Khoto and its scientific value Guixi Liu1*, Wurheng2, Khasbagan1,2,3*, Yanying Zhang1 and Shirong Guo1 1 Institute for the History of Science and Technology, Inner Mongolia Normal University, Hohhot, 010022, China. E-mail: [email protected], [email protected] 2 College of Life Science and Technology, Inner Mongolia Normal University, Hohhot, 010022, China. 3 Key Laboratory Breeding Base for Biodiversity Conservation and Sustainable Use of Colleges and Universities in Inner Mongolia Autonomous Region, China. * the corresponding author 1 Abstract Background: There is a plentiful amount of local knowledge on plants hidden in the literature of foreign exploration to China in modern history. Mongolia and Amdo and the Dead City of Khara- Khoto (MAKK) is an expedition record on the sixth scientific expedition to northwestern China (1907-1909) initiated by P. -

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Karyological Studies on Iris Japonica Thunb. and Its Allies1) 2) by K
180 Cytologia 10 Karyological Studies on Iris japonica Thunb. and Its Allies1) 2) By K. Yasui Tokyo Imperial University (With 8 text figures) Receic ed MTV 28, 1939 Introduction There are several karyological investigations on Iris japonica. KAZAO (1928) considered the sporophyte of this species as an auto triploid having 54 chromosomes. But SIMONET (1932, 1934) re ported I. japonica as a diploid plant with 34 chromosomes in its root-tip cells, and considered I. japonica var aphrodite as a hyper triploid and interpreted the chromosome constitution as 2n=51+3, the latter 3 chromosomes being considered as derived from 3 of 51 chromosomes in I. japonica by fragmentation which occurred att their median constrictions. The karyological investigation in I. japonica and the other 2 allied species, which were kindly put at my deposal by Dr. S. IMA MURA of Kyoto Imp. University, gave me some results different from those of the previous authors as is described below. Material and Method In the fixing of the root-tip cells both NAVASHIN's and LEWITSKY's solutions were used. When fixed in the latter, the chromosomes were found longer and slender than when fixed in the former solution, but there was no essential difference in the distribu tion of chromosomes in the equatorial plate. NEWTON'S gentian violet staining was generally adopted. Materials for the study of meiosis were fixed mostly with NAVASHIN'S fluid and stained with HEIDENHAIN'S iron-alum haematoxylin. Acetocarmine smears were also tried in the study of the pollen mother cells. Drawings were made with a camera lucida. -
Scanned Document
~ l ....... , .,. ... , •• 1 • • .. ,~ . · · . , ' .~ . .. , ...,.,, . ' . __.... ~ •"' --,~ ·- ., ......... J"'· ·····.-, ... .,,,.."" ............ ,... ....... .... ... ,,··~·· ....... v • ..., . .......... ,.. •• • ..... .. .. ... -· . ..... ..... ..... ·- ·- .......... .....JkJ(o..... .. I I ..... D · . ··.·: \I••• . r .• ! .. THE SPECIES IRIS STUDY GROUP OF THE AMERICAN IRIS SOCIETY \' -... -S:IGNA SPECIES IRIS GROUP OF NORTH AMERICA APRIL , 1986 NO. 36 OFFICERS CHAIRMAN: Elaine Hulbert Route 3, Box 57 Floyd VA 24091 VICE--CHAI.RMAN: Lee Welsr, 7979 W. D Ave. ~<alamazoo MI 4900/i SECRETARY: Florence Stout 150 N. Main St. Lombard, IL 6014~ TREASURER: Gene Opton 12 Stratford Rd. Berkelew CA 9470~ SEED EXCHANGE: Merry&· Dave Haveman PO Box 2054 Burling~rne CA 94011 -RO:E,IN DIRECTOR: Dot HuJsak 3227 So. Fulton Ave. Tulsc1, OK 74135 SLIDE DIRECTO~: Colin Rigby 2087 Curtis Dr . Penngrove CA 9495~ PUBLICATIONS SALES: Alan McMu~tr1e 22 Calderon Crescent Willowdale, Ontario, Canada M2R 2E5 SIGNA EDITOR : .Joan Cooper 212 W. Count~ Rd. C Roseville MN 55113 SIGNA PUBLISl-!ER:. Bruce Richardson 7 249 Twenty Road, RR 2 Hannon, Ontario, Canada L0R !Pe CONTENTS--APRIL, 1986--NO. 36 CHAIRMAN'S MESSAGE Elaine HL\l ber t 1261 PUBLICATI~NS AVAILABLE Al an McMwn tr ie 12c)1 SEED EXCHANGE REPORT David & Merry Haveman 1262 HONORARY LIFE MEMBERSHIPS El a ine? HLtlbert 1263 INDEX REPORTS Eric Tankesley-Clarke !263 SPECIES REGISTRATIONS--1985 Jean Witt 124-4' - SLIDE COLLECTION REPORT Col in Rigby 1264 TREASURER'S REPORT Gene (>pton 1264, NOMINATING COMMITTEE REPORT Sharon McAllister 1295 IRIS SOURCES UPDATE Alan McMurtrie 1266 QUESTIONS PLEASE '-Toan Cooper 1266 NEW TAXA OF l,P,IS L . FROM CHINA Zhao Yu·-· tang 1.26? ERRATA & ADDENDA ,Jim Rhodes 1269 IRIS BRAI\ICHil\iG IN TWO MOl~E SPECIES Jean Witt 1270 TRIS SPECIES FOR SHALLOW WATER Eberhard Schuster 1271 JAPANESE WILD IRISES Dr. -
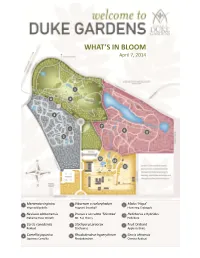
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

These De Doctorat De L'universite Paris-Saclay
NNT : 2016SACLS250 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de doctorat (Biologie) Par Mlle Nour Abdel Samad Titre de la thèse (CARACTERISATION GENETIQUE DU GENRE IRIS EVOLUANT DANS LA MEDITERRANEE ORIENTALE) Thèse présentée et soutenue à « Beyrouth », le « 21/09/2016 » : Composition du Jury : M., Tohmé, Georges CNRS (Liban) Président Mme, Garnatje, Teresa Institut Botànic de Barcelona (Espagne) Rapporteur M., Bacchetta, Gianluigi Università degli Studi di Cagliari (Italie) Rapporteur Mme, Nadot, Sophie Université Paris-Sud (France) Examinateur Mlle, El Chamy, Laure Université Saint-Joseph (Liban) Examinateur Mme, Siljak-Yakovlev, Sonja Université Paris-Sud (France) Directeur de thèse Mme, Bou Dagher-Kharrat, Magda Université Saint-Joseph (Liban) Co-directeur de thèse UNIVERSITE SAINT-JOSEPH FACULTE DES SCIENCES THESE DE DOCTORAT DISCIPLINE : Sciences de la vie SPÉCIALITÉ : Biologie de la conservation Sujet de la thèse : Caractérisation génétique du genre Iris évoluant dans la Méditerranée Orientale. Présentée par : Nour ABDEL SAMAD Pour obtenir le grade de DOCTEUR ÈS SCIENCES Soutenue le 21/09/2016 Devant le jury composé de : Dr. Georges TOHME Président Dr. Teresa GARNATJE Rapporteur Dr. Gianluigi BACCHETTA Rapporteur Dr. Sophie NADOT Examinateur Dr. Laure EL CHAMY Examinateur Dr. Sonja SILJAK-YAKOVLEV Directeur de thèse Dr. Magda BOU DAGHER KHARRAT Directeur de thèse Titre : Caractérisation Génétique du Genre Iris évoluant dans la Méditerranée Orientale. Mots clés : Iris, Oncocyclus, région Est-Méditerranéenne, relations phylogénétiques, status taxonomique. Résumé : Le genre Iris appartient à la famille des L’approche scientifique est basée sur de nombreux Iridacées, il comprend plus de 280 espèces distribuées outils moléculaires et génétiques tels que : l’analyse de à travers l’hémisphère Nord. -

Starch Research Page URL: Last Updated: 14-April-2004 / Du Ethnobotanical Leaflets Starch Research Page
Journal Contents Back Issues Book Reviews Research Notes Careers Meetings Botany Resources Why study starch? The long and short of it is that size, shape, and optical and chemical properties of starch are useful in the determination of taxonomic species. Starch characteristics can be used in place of, or in combination with other features of the plant to facilitate the identification of macro archaeological plant remains, or even small pieces of modern plant materials which for one reason or the other are lacking the traditional "key" taxonomic characters. As in the case of pollen and phytolith analyses, the study of fossil starch grains can help the ethnobotanist to better understand the diet of ancient human cultures. The taxonomic usefulness of starch was long ago noted by Edward Tyson Reichert (1913), whose monumental work on "The Differentiation and Specificity of Starches in Relation to Genera, Species, Etc." has today become a classic in its field. He writes: "It must have been recognized by Leeuwenhoek, and by many of the investigators of the earliest part of the last century, that starches from different sources are not morphologically identical, but if so it does not seem to have attracted any particular attention until the investigations of Fritzsche (Ann. d. Physik. u. Chernie, 1834, xxxii, 129). Fritzsche described the starches obtained from a variety of plants. He noted not only that the starches from different sources were different, but also that often the form was so characteristic as to determine the plant, or, at least, indicate the genus and family from which the specimen was obtained. -

Vol. 49 Valencia, X-2011 FLORA MONTIBERICA
FLORA MONTIBERICA Publicación periódica especializada en trabajos sobre la flora del Sistema Ibérico Vol. 49 Valencia, X-2011 FLORA MONTIBERICA Publicación independiente sobre temas relacionados con la flora y la vegetación (plantas vasculares) de la Península Ibérica, especialmente de la Cordillera Ibérica y tierras vecinas. Fundada en diciembre de 1995, se publican tres volúmenes al año con una periodicidad cuatrimestral. Editor y Redactor general: Gonzalo Mateo Sanz. Jardín Botánico. Universidad de Valencia. C/ Quart, 80. E-46008 Valencia. Redactores adjuntos: Javier Fabado Alós. Redactor página web y editor adjunto: José Luis Benito Alonso. Edición en Internet: www.floramontiberica.org Flora Montiberica.org es la primera revista de botánica en español que ofrece de forma gratuita todos sus contenidos a través de la red. Consejo editorial: Antoni Aguilella Palasí (Universidad de Valencia) Juan A. Alejandre Sáenz (Herbarium Alejandre, Vitoria) Vicente J. Arán Redó (Consejo Superior de Investigaciones Científicas, Madrid) Manuel Benito Crespo Villalba (Universidad de Alicante) José María de Jaime Lorén (Universidad Cardenal Herrera-CEU, Moncada) Emilio Laguna Lumbreras ((Departamento de Medio Ambiente. Gobierno de la Comunidad Valenciana) Pedro Montserrat Recoder (Consejo Superior de Investigaciones Científicas, Jaca). Edita: Flora Montiberica. Valencia (España). ISSN: 1138-5952 – ISSN edición internet: 1988-799X. Depósito Legal: V-5097-1995. Portada: Ophioglossum azoricum C. Presl, procedente de Sotorribas (Cuenca). Véase pág. 36 de este número. Flora Montiberica 49: 3-5 (X-2011). ISSN 1988-799X NUEVA LOCALIDAD VALENCIANA DE PUCCINELLIA HISPANICA JULIÀ & J. M. MONTSERRAT (POACEAE) P. Pablo FERRER GALLEGO1 & Roberto ROSELLÓ GIMENO2 1Servicio de Biodiversidad, Centro para la Investigación y la Experimentación Forestal de la Generalitat Valenciana (CIEF). -

Establishment of an Efficient in Vitro Propagation System for Iris Sanguinea
www.nature.com/scientificreports OPEN Establishment of an efcient in vitro propagation system for Iris sanguinea Received: 13 June 2018 Ling Wang1, Yu Du1, Md. Mahbubur Rahman2, Biao Tang1, Li-Juan Fan1 & Aruna Kilaru 2 Accepted: 2 November 2018 Iris sanguinea is a perennial fowering plant that is typically cultivated through seeds or bulbs. However, Published: xx xx xxxx due to limitations in conventional propagation, an alternate regeneration system using seeds was developed. The protocol included optimization of sterilization, stratifcation and scarifcation methods as iris seeds exhibit physiological dormancy. In addition to chlorine-based disinfection, alkaline or heat treatment was used to break seed dormancy and reduce contamination. When seeds were soaked in water at 80 °C overnight, and sterilized with 75% EtOH for 30 s and 4% NaOCl solution for 20 minutes, contamination was reduced to 10% and a 73.3% germination was achieved. The germinated seedlings with 2-3 leaves and radicle were used as explants to induce adventitious buds. The optimal MS medium with 0.5 mg L−1 6-benzylaminopurine, 0.2 mg L−1 NAA, and 1.0 mg L−1 kinetin resulted in 93.3% shoot induction and a proliferation coefcient of 5.30. Medium with 0.5 mg L−1 NAA achieved 96.4% rooting of the adventitious shoots. The survival rate was more than 90% after 30 days growth in the cultivated matrix. In conclusion, a successful regeneration system for propagation of I. sanguinea was developed using seeds, which could be utilized for large-scale propagation of irises of ecological and horticultural importance. -

Iris Pallida This Species Iris, Also Know As Zebra Iris, Sweet Iris, Or Dalmatian Iris, Is a Very Old Garden Plant
A Horticulture Information article from the Wisconsin Master Gardener website, posted 21 May 2007 Iris pallida This species iris, also know as Zebra Iris, Sweet Iris, or Dalmatian Iris, is a very old garden plant. Native to rocky areas of northern Italy and the Eastern Mediterranean (including Dalmatia, a province of Croatia, hence one of the common names), it was one of the primary species used in the development of the tall bearded iris. Its dried root (along with other species of Iris) is a source of orris root powder that was used medicinally or for its supposed magical and alchemical properties in medieval times, as well as a per- fume and potpourri fi xative for many centuries. It may take several years of drying for the root to fully develop its fragrance. Orris oil, derived from the fresh root, is used as a fl avoring Iris pallida ‘Aurea-variegata’ in bud in soft drinks, candies and chewing gum. The low clumps of sword-like leaves remain nearly evergreen in mild winter climates, but in Iris pallida ‘Argentea Variegata’ at cold areas the plant dies back to the ground like RHS Garden Wisley in England. other irises do. The thick foliage of the cultivars commonly offered as garden plants has elegant vertical stripes of blue-green and either silvery-white (‘Alba-vareigata’ or ‘Argentea Variegata’) or creamy yel- low to pale gold (“Aurea-variegata’ or ‘Variegata’), depending on the cultivar. The Iris pallida foliage. foliage of the species is not quite as attractive, being a solid bluish-green. The cultivars are grown primarily for their attractive striped leaves, although they do produce pretty lav- ender-blue fl owers with small yellow beards in early summer on 3 foot tall scapes.