IL-13 Predicts the Need for Mechanical Ventilation in COVID-19 Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modulation of the Inflammatory Response After Spinal Cord Injury
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en MODULATION OF THE INFLAMMATORY RESPONSE AFTER SPINAL CORD INJURY Presented by Jesús Amo Aparicio ACADEMIC DISSERTATION To obtain the degree of PhD in Neuroscience by the Universitat Autònoma de Barcelona 2019 Directed by Dr. Rubèn López Vales Tutorized by Dr. Xavier Navarro Acebes INDEX SUMMARY Page 7 INTRODUCTION Page 13 - Spinal cord Page 15 - Spinal cord injury Page 17 - Incidence and causes Page 18 - Types of SCI Page 18 - Biological events after SCI Page 20 - Studying SCI Page 24 - Animal models Page 24 - Lesion models Page 24 - Current therapies for SCI Page 25 - Basic principles of the immune system Page 27 - Innate immune response Page 27 - Adaptive immune response Page 28 - Inflammatory response Page 29 - Inflammatory response after SCI Page 30 - Modulation of injury environment Page 36 - Interleukin 1 Page 36 - Interleukin 37 Page 40 - Interleukin 13 Page 44 OBJECTIVES Page 47 MATERIALS AND METHODS Page 51 -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

Interleukin 13, a T-Cell-Derived Cytokine That Regulates Human Monocyte and B-Cell Function A
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 3735-3739, April 1993 Immunology Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function A. N. J. MCKENZIE*, J. A. CULPEPPER*, R. DE WAAL MALEFYTt, F. BRItREt, J. PUNNONENt, G. AVERSAt, A. SATO*, W. DANG*, B. G. COCKSt, S. MENON*, J. E. DE VRIESt, J. BANCHEREAUt, AND G. ZURAWSKI*§ Departments of *Molecular Biology and tHuman Immunology, DNAX Research Institute of Molecular and Cellular Biology, 901 California Avenue, Palo Alto, CA 94304-1104; and tSchering-Plough, Laboratory for Immunological Research, 27 chemin des peupliers, B.P.11, 69572 Dardilly, France Communicated by Avram Goldstein, December 28, 1992 (received for review November 2, 1992) ABSTRACT We have isolated the human cDNA homo- Tonsillar B cells were prepared as described (5). Flow logue of a mouse helper T-cell-specific cDNA sequence, called cytometric analysis following staining with fluorescein- P600, from an activated human T-cell cDNA library. The labeled anti-CD19, -CD20, -CD14 (Becton Dickinson), -CD2, human cDNA encodes a secreted, mainly unglycosylated, pro- and -CD3 (Immunotech, Luminy, France) showed that the tein with a relative molecular mass of -10,000. We show that purity of this B-cell population was >95%. the human and mouse proteins cause extensive morphological Splenic B cells were purified from a normal human spleen, changes to human monocytes with an associated up-regulation obtained from a transplant donor, by negative fluorescence- of major histocompatibility complex class II antigens and the activated cell sorting (FACStar Plus, Becton Dickinson); low-affinity receptor for immunoglobulin E (FcERII or CD23). -

Molecular Signatures Differentiate Immune States in Type 1 Diabetes Families
Page 1 of 65 Diabetes Molecular signatures differentiate immune states in Type 1 diabetes families Yi-Guang Chen1, Susanne M. Cabrera1, Shuang Jia1, Mary L. Kaldunski1, Joanna Kramer1, Sami Cheong2, Rhonda Geoffrey1, Mark F. Roethle1, Jeffrey E. Woodliff3, Carla J. Greenbaum4, Xujing Wang5, and Martin J. Hessner1 1The Max McGee National Research Center for Juvenile Diabetes, Children's Research Institute of Children's Hospital of Wisconsin, and Department of Pediatrics at the Medical College of Wisconsin Milwaukee, WI 53226, USA. 2The Department of Mathematical Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI 53211, USA. 3Flow Cytometry & Cell Separation Facility, Bindley Bioscience Center, Purdue University, West Lafayette, IN 47907, USA. 4Diabetes Research Program, Benaroya Research Institute, Seattle, WA, 98101, USA. 5Systems Biology Center, the National Heart, Lung, and Blood Institute, the National Institutes of Health, Bethesda, MD 20824, USA. Corresponding author: Martin J. Hessner, Ph.D., The Department of Pediatrics, The Medical College of Wisconsin, Milwaukee, WI 53226, USA Tel: 011-1-414-955-4496; Fax: 011-1-414-955-6663; E-mail: [email protected]. Running title: Innate Inflammation in T1D Families Word count: 3999 Number of Tables: 1 Number of Figures: 7 1 For Peer Review Only Diabetes Publish Ahead of Print, published online April 23, 2014 Diabetes Page 2 of 65 ABSTRACT Mechanisms associated with Type 1 diabetes (T1D) development remain incompletely defined. Employing a sensitive array-based bioassay where patient plasma is used to induce transcriptional responses in healthy leukocytes, we previously reported disease-specific, partially IL-1 dependent, signatures associated with pre and recent onset (RO) T1D relative to unrelated healthy controls (uHC). -

Anti-Alarmins in Asthma: Targeting the Airway Epithelium with Next-Generation Biologics
REVIEW | ASTHMA Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics Celeste M. Porsbjerg1, Asger Sverrild1, Clare M. Lloyd 2, Andrew N. Menzies-Gow3 and Elisabeth H. Bel4 Affiliations: 1Dept of Respiratory Medicine, Bispebjerg Hospital, Copenhagen, Denmark. 2National Heart and Lung Institute, Imperial College London, London, UK. 3Royal Brompton Hospital, London, UK. 4Dept of Respiratory Medicine, Amsterdam University Medical Centre, University of Amsterdam, Amsterdam, The Netherlands. Correspondence: Celeste M. Porsbjerg, Dept of Respiratory Medicine, Bispebjerg Hospital, Bispebjerg Bakke 23, 2400 Copenhagen, Denmark. E-mail: [email protected] @ERSpublications Blocking epithelial alarmins, upstream mediators triggered early in the asthma inflammatory response that orchestrate broad inflammatory effects, is a promising alternative approach to asthma treatment, which may be effective in a broad patient population https://bit.ly/2zqoXAw Cite this article as: Porsbjerg CM, Sverrild A, Lloyd CM, et al. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J 2020; 56: 2000260 [https://doi.org/10.1183/ 13993003.00260-2020]. ABSTRACT Monoclonal antibody therapies have significantly improved treatment outcomes for patients with severe asthma; however, a significant disease burden remains. Available biologic treatments, including anti-immunoglobulin (Ig)E, anti-interleukin (IL)-5, anti-IL-5Rα and anti-IL-4Rα, reduce exacerbation rates in study populations by approximately 50% only. Furthermore, there are currently no effective treatments for patients with severe, type 2-low asthma. Existing biologics target immunological pathways that are downstream in the type 2 inflammatory cascade, which may explain why exacerbations are only partly abrogated. For example, type 2 airway inflammation results from several inflammatory signals in addition to IL-5. -

IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells
cells Article IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells Fridolin Franke 1,2, Greg A. Kirchenbaum 1 , Stefanie Kuerten 2 and Paul V. Lehmann 1,* 1 Research & Development Department, Cellular Technology Limited, Shaker Heights, OH 44122, USA; [email protected] (F.F.); [email protected] (G.A.K.) 2 Institute of Anatomy and Cell Biology, Friedrich-Alexander University Erlangen-Nürnberg, 91054 Erlangen, Germany; [email protected] * Correspondence: [email protected]; Tel.: +1-216-965-6311 Received: 3 December 2019; Accepted: 12 February 2020; Published: 13 February 2020 Abstract: Detection of antigen-specific memory B cells for immune monitoring requires their activation, and is commonly accomplished through stimulation with the TLR7/8 agonist R848 and IL-2. To this end, we evaluated whether addition of IL-21 would further enhance this TLR-driven stimulation approach; which it did not. More importantly, as most antigen-specific B cell responses are T cell-driven, we sought to devise a polyclonal B cell stimulation protocol that closely mimics T cell help. Herein, we report that the combination of agonistic anti-CD40, IL-4 and IL-21 affords polyclonal B cell stimulation that was comparable to R848 and IL-2 for detection of influenza-specific memory B cells. An additional advantage of anti-CD40, IL-4 and IL-21 stimulation is the selective activation of IgM+ memory B cells, as well as the elicitation of IgE+ ASC, which the former fails to do. Thereby, we introduce a protocol that mimics physiological B cell activation through helper T cells, including induction of all Ig classes, for immune monitoring of antigen-specific B cell memory. -

IL-5 Regulate Airways Hyperreactivity Integrated Signals Between IL-13
Integrated Signals Between IL-13, IL-4, and IL-5 Regulate Airways Hyperreactivity Dianne C. Webb, Andrew N. J. McKenzie, Aulikki M. L. Koskinen, Ming Yang, Joërg Mattes and Paul S. Foster This information is current as of September 25, 2021. J Immunol 2000; 165:108-113; ; doi: 10.4049/jimmunol.165.1.108 http://www.jimmunol.org/content/165/1/108 Downloaded from References This article cites 48 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/165/1/108.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 25, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Integrated Signals Between IL-13, IL-4, and IL-5 Regulate Airways Hyperreactivity Dianne C. Webb,* Andrew N. J. McKenzie,† Aulikki M. L. Koskinen,* Ming Yang,* Joe¨rg Mattes,* and Paul S. Foster1* In this investigation, we have examined the integrated relationship between IL-13, IL-4, and IL-5 for the development of airways hyperreactivity (AHR) in a model of asthma in BALB/c mice. -
IL-13) Signaling in Inflammatory Diseases
Interleukin-13 (IL-13) Signaling in Inflammatory Diseases About IL-13 Signaling IL-13 is a type of small protein, known as a cytokine, that plays an important role in the immune system's response to an invading parasite or an allergen.1 IL-13 exerts its effects by binding to receptors called IL-13Rα1 and IL-13Rα2 found on cell surfaces. When IL-13 binds to its receptors, it sets off a series of pathways that lead to cell-to-cell communication, known as signaling, triggering normal inflammatory processes that protect the body.2,3 IL-13’s Role in Inflammatory Diseases Though IL-13 is a normal part of the human immune response, it can be dysregulated. Signaling from IL-13Rα1 can contribute to allergic inflammation (an inappropriate inflammatory response) and chronic inflammation can result in fibrosis (tissue damage or scarring). In addition, signaling from IL-13Rα2 may contribute directly 1,4–6 Eosinophil to fibrosis. Allergic inflammation is regulated by cytokines, including IL-13, that play a key role in the recruitment of inflammatory cells to sites of inflammation.1 Eosinophils are one such type of inflammatory cell. While present in low numbers in healthy individuals, larger numbers of eosinophils can contribute to the development of multiple inflammatory diseases, such as eosinophilic esophagitis, atopic dermatitis, eosinophilic and allergic asthma, and chronic rhinosinusitis with nasal polyps.3 IL-13 has been found to be elevated in patients with eosinophilic disorders, with IL-13 signaling contributing to the recruitment, activation, and survival of eosinophils.3 Research Implications Researchers are investigating potential ways to inhibit IL-13 signaling to prevent inflammation and fibrosis.7,8 One strategy involves blocking IL-13 before it can bind to receptors on cell surfaces, preventing the activation of subsequent signaling pathways.7,8 At Bristol Myers Squibb, we continue to pioneer novel approaches by leveraging our depth of experience to advance research in immune-mediated and inflammatory diseases. -

Elevated Serum Levels of Interleukin-13 and Interleukin-17A in Pediatric Asthma
Sys Rev Pharm 2020;11(9):229-233 A multifaceted review journal in the field of pharmacy Elevated serum levels of Interleukin-13 and Interleukin-17A in Pediatric Asthma Sawsan M. Jabbar AL-Hasnawi1*, Abeer Thaher Naji AL-Hasnawi1 1Department of Medical Microbiology/College of Medicine of Kerbala University, Kerbala, Iraq. Correspondence to: Sawsan M. Jabbar AL-Hasnawi Email: [email protected] ABSTRACT Background: Pediatric asthma is a chronic serious disease, which is difficult Keywords: IL-13, IL-17A, Th2, Th17, Pediatric asthma to diagnose with heterogeneous etiology. Till now it is not clear how T helper (Th)2 & Th17 pathways interact in pathophysiology of asthma, the dominant Correspondence: pathway can lead to different phenotypes.Th2-low (non Th2) which is Sawsan M. Jabbar AL-Hasnawi mediated mainly by Th17, Th17-high and Th2-high type mediated by Th2 cell Department of Medical Microbiology/College of Medicine of Kerbala subset of lymphocytes are two different phenotypes of asthma. University, Kerbala, Iraq. Objectives: The present study focused on interleukin-17A (IL-17A), a major Correspondence to: Sawsan M. Jabbar AL-Hasnawi Th17 cytokine and interleukin-13 (IL-13) a cytokine secreted by Th2 cells Email: [email protected] trying to elucidate their combined role in airway inflammation. Method: This is a case-controlled study include 149 children with a mean age of 1-15 years, 74 children with asthma and 75 control healthy subjects performed at asthma clinic outpatient during October 2019 through December 2019. Blood samples from all participants subjected to analysis by ELISA for measurement of IL-17A & IL-13 serum levels. -
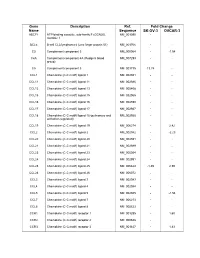
Supplementary Data
Gene Description Ref. Fold Change Name Sequence SK-OV-3 OVCAR-3 ABCF1 ATP-binding cassette, sub-family F (GCN20), NM_001090 - - member 1 BCL6 B-cell CLL/lymphoma 6 (zinc finger protein 51) NM_001706 - - C3 Complement component 3 NM_000064 - -1.54 C4A Complement component 4A (Rodgers blood NM_007293 - - group) C5 Complement component 5 NM_001735 13.74 - CCL1 Chemokine (C-C motif) ligand 1 NM_002981 - - CCL11 Chemokine (C-C motif) ligand 11 NM_002986 - - CCL13 Chemokine (C-C motif) ligand 13 NM_005408 - - CCL15 Chemokine (C-C motif) ligand 15 NM_032965 - - CCL16 Chemokine (C-C motif) ligand 16 NM_004590 - - CCL17 Chemokine (C-C motif) ligand 17 NM_002987 - - CCL18 Chemokine (C-C motif) ligand 18 (pulmonary and NM_002988 - - activation-regulated) CCL19 Chemokine (C-C motif) ligand 19 NM_006274 - 2.42 CCL2 Chemokine (C-C motif) ligand 2 NM_002982 - -2.23 CCL20 Chemokine (C-C motif) ligand 20 NM_004591 - - CCL21 Chemokine (C-C motif) ligand 21 NM_002989 - - CCL23 Chemokine (C-C motif) ligand 23 NM_005064 - - CCL24 Chemokine (C-C motif) ligand 24 NM_002991 - - CCL25 Chemokine (C-C motif) ligand 25 NM_005624 -1.49 2.59 CCL26 Chemokine (C-C motif) ligand 26 NM_006072 - - CCL3 Chemokine (C-C motif) ligand 3 NM_002983 - - CCL4 Chemokine (C-C motif) ligand 4 NM_002984 - - CCL5 Chemokine (C-C motif) ligand 5 NM_002985 - -1.54 CCL7 Chemokine (C-C motif) ligand 7 NM_006273 - - CCL8 Chemokine (C-C motif) ligand 8 NM_005623 - - CCR1 Chemokine (C-C motif) receptor 1 NM_001295 - 1.60 CCR2 Chemokine (C-C motif) receptor 2 NM_000648 - - CCR3 Chemokine (C-C -

Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Rα2
Published January 5, 2018, doi:10.4049/jimmunol.1701256 The Journal of Immunology Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Ra2 Jennifer Duffen,* Melvin Zhang,* Katherine Masek-Hammerman,† Angela Nunez,‡ Agnes Brennan,* Jessica E. C. Jones,x Jeffrey Morin,‡ Karl Nocka,* and Marion Kasaian* In obesity, IL-13 overcomes insulin resistance by promoting anti-inflammatory macrophage differentiation in adipose tissue. En- dogenous IL-13 levels can be modulated by the IL-13 decoy receptor, IL-13Ra2, which inactivates and depletes the cytokine. In this study, we show that IL-13Ra2 is markedly elevated in adipose tissues of obese mice. Mice deficient in IL-13Ra2 had high expression of IL-13 response markers in adipose tissue, consistent with increased IL-13 activity at baseline. Moreover, exposure to the type 2 cytokine-inducing alarmin, IL-33, enhanced serum and tissue IL-13 concentrations and elevated tissue eosinophils, macrophages, and type 2 innate lymphoid cells. IL-33 also reduced body weight, fat mass, and fasting blood glucose levels. Strikingly, however, the IL-33–induced protection was greater in IL-13Ra2–deficient mice compared with wild-type littermates, and these changes were largely attenuated in mice lacking IL-13. Although IL-33 administration improved the metabolic profile in the context of a high fat diet, it also resulted in diarrhea and perianal irritation, which was enhanced in the IL-13Ra2–deficient mice. Weight loss in this group was associated with reduced food intake, which was likely related to the gastrointestinal effects. These findings outline both potentially advantageous and deleterious effects of a type 2–skewed immune response under conditions of metabolic stress, and identify IL-13Ra2 as a critical checkpoint in adipose tissues that limits the protective effects of the IL-33/ IL-13 axis in obesity. -

4, IL-13, and IL-17A Differentially Affect the Profibrotic and Proinflammatory Functions of Fibrocytes from Asthmatic Patients
ARTICLES nature publishing group See COMMENTARY page XX Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients A B e l l i n i 1 , M A M a r i n i 2 , L B i a n c h e t t i 1 , M B a r c z y k 1 , M S c h m i d t 1 a n d S M a t t o l i 1 Fibrocytes contribute to the fibrotic changes most frequently observed in forms of asthma where inflammation is driven by T helper type 2 (Th2) cells. The mechanisms that regulate the profibrotic function of asthmatic fibrocytes are largely unknown. We isolated circulating fibrocytes from patients with allergen-exacerbated asthma, who showed the presence of fibrocytes, together with elevated concentrations of interleukin (IL)-4 and IL-13 and slightly increased concentrations of the Th17 cell-derived IL-17A, in induced sputum. Fibrocytes stimulated with IL-4 and IL-13 produced high levels of collagenous and non-collagenous matrix components and low levels of proinflammatory cytokines. Conversely, fibrocytes stimulated with IL-17A proliferated and released proinflammatory factors that may promote neutrophil recruitment and airway hyperresponsiveness. IL-17A also indirectly increased -smooth muscle actin but not collagen expression in fibrocytes. Thus, fibrocytes may proliferate and express a predominant profibrotic or proinflammatory phenotype in asthmatic airways depending on the local concentrations of Th2- and Th17-derived cytokines. INTRODUCTION tion. 3,6,7,9,10 This thickening of the subepithelial reticular sheet