Nutrition Support for Athletic Performance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2013 Summer Deaflympics
www.ukdeafsport.co.uk UK Deaf Sport Register Charity No: 1111776 More deaf people participating and reaching their their reaching and participating people deaf More Project Lead & Chef deMission Lead &Chef Project Gordon Hay & Fiona Brookes Hay Gordon full potential in sportin potential full 2013 Summer 2013 Deaflympics www.ukdeafsport.co.uk UK Deaf Sport Register Charity No: 1111776 More deaf people participating and reaching their their reaching and participating people deaf More full potential in sportin potential full Taipei to Sofia via Athens • Athens was chosen as venue for 2013 – Due to financial restrictions in Greece; the city withdrew www.ukdeafsport.co.uk Charity No: 1111776 Register • Hungary stepped in as potential venue – Cost became impossible; ICSD seeks alternative • Sofia expressed interest and confirmed as venue UK Deaf Sport Deaf UK More deaf people participating and reaching their full potential in sport www.ukdeafsport.co.uk UK Deaf Sport Register Charity No: 1111776 More deaf people participating and reaching their their reaching and participating people deaf More full potential in sportin potential full Sofia 2013 • Confirmed venue and signed commitment to proceed with the preparations for the Games – 10 months before the Opening Ceremony date www.ukdeafsport.co.uk Charity No: 1111776 Register • Challenges; – Information – Selections – Logistics UK Deaf Sport Deaf UK – Finances More deaf people participating and reaching their full potential in sport www.ukdeafsport.co.uk UK Deaf Sport Register Charity No: 1111776 -
2013 Summer Deaflympics (Taekwondo)
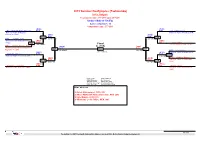
2013 Summer Deaflympics (Taekwondo) Sofia, Bulgaria Tournament date: 27-7-2013 upto 28-7-2013 Seniors Male A -58 (Fly) Active competitors: 10 Competition date: 27-7-2013 (RUS) (TUR) Aliev, Murtazali Abdusalamovich B/37 B/61 Guler, Muhammed National Team of Russian National Team of Turkey (TUR) Federation (RUS) (RUS) (TUR) 113 B/37 B/61 115 B/21 Abdrash, Ayan (18-6 PTG) (18-10 PTF) National Team of Kazakhstan (KAZ) (GRE) (KAZ) 101 R/21 R/5 Liotsos, Pavlos (11-7 PTG) Final Greece National team (GRE) R/13 Borhani Peikani, Ahmadreza (RUS) 61 (TUR) (TUR) National Team of Iran (Islamic B/37 R/61 Republic of) (IRI) 125 131 126 (8-0 RSC) (2-1 PTF) B/27 Kim, Minjae (UKR) (13-13 RSC) (KOR) National Team of South Korea Salamatin, Mykolai B/70 B/27 102(KOR) National Team of Ukraine (UKR) (16-4 PTG) R/76 (MEX) (KOR) Khidirov, Davron National Team of Uzbekistan 114 R/33 R/27 116 (3-8 PTF) (12-0 PTG) (UZB) (MEX) (HKG) Villarreal, Louis Felipe R/33 R/9 Ho, Nim Ching National Team of Mexico (MEX) National Team of Hong Kong (HKG) Result legend: (PUN) Punishment (WDR) Withdrawal (KO ) Knock out (DSQ) Disqualified (SDP) Sudden death (RSC) Ref. stop contest (PTG) Points win (gap) (SUP) Superiority (PTF) Points win (normal) Prize winners: 1 Guler, Muhammed, TUR (61) 2 Aliev, Murtazali Abdusalamovich, RUS (37) 3 Kim, Minjae, KOR (27) 3 Villarreal, Louis Felipe, MEX (33) 1st rnd QF SF Final SF QF 1st rnd TaekoPlan (c) 2013 SenSoft Automation Alphen aan den Rijn, Netherlands (www.taekoplan.nl) 2013 Summer Deaflympics (Taekwondo) Sofia, Bulgaria Tournament date: -

Dossier De Presse Deaflympics 2013X
Dossier de presse Deaflympics 2013 Avant-propos Comité Olympique et Interfédéral Belge (COIB) Mesdames, Messieurs, C’est avec grand plaisir que nous sommes ici ce soir pour accueillir les athlètes et les accompagnateurs de la délégation belge qui participer ont du 26 juillet au 4 août au x Deaflympics à Sofia en Bulgarie. 5 athlètes et 9 officiels représenteront les couleurs de la Belgique. Il n’y a pas un an que les Jeux Olympiques de Londres ont eu lieu , que déjà l’été 2013 compte un bon nombre de compétitions multidisciplinaires de haut niveau. Je pense ainsi aux Jeux Mondiaux à Cali en Colombie, au Festival Olympique de la Jeunesse Européenne à Utrecht aux Pays -Bas et bien entendu aux Deaflympics. Nous pouvons être fiers que notre pays joue et a joué un rôle important dans beaucoup de ces événements sportifs tant de par sa participation que de par les structures. Je p ense à Jacques Rogge qui prendra sa retraite en tant que président du Comité International Olympique fin de cette année et aussi à Antoine Dresse, qui en 1924 était à l’origine des Deaflympics, une des plus anciennes organisations pour athlètes avec un handicap. Dans un instant, je donnerai la parole à Paul De Weerdt, le président du Belgian Deaf sport Committee. Il nous dira tout sur les Deaflympics à Sofia et sur la composition de la délégation belge. Les Jeux Olympiques pour sourds se sont développés en un événement sportif de haut niveau. Des milliers d’athlètes du monde entier démontrent avec ferveur que les sourds et les malentendants ont mérité une place dans notre société. -

Matthew P. Brancaleone, PT, DPT, SCS, AT, CSCS [email protected]
Matthew P. Brancaleone, PT, DPT, SCS, AT, CSCS [email protected] EDUCATION The Ohio State University, Columbus, OH 2016-Present Doctorate of Philosophy in Health and Rehabilitation Sciences Advisors: John Bolte, Ph.D. and Laura Boucher, Ph.D., AT The Ohio State University, Columbus, OH 2014-2015 Sports Physical Therapy Residency The Ohio State University Sports Medicine Central Michigan University, Mt. Pleasant, MI 2011-2014 Doctor of Physical Therapy Advisor: Elaine Filusch Betts, PT, Ph.D., FACSM Central Michigan University, Mt. Pleasant, MI 2006-2010 Bachelor of Science – Athletic Training; American Sign Language Advisor: René Revis Shingles, Ph.D., AT, ATC LICENSURE Licensed Physical Therapist 2014-Present State of Ohio - 014937 Licensed Athletic Trainer 2014-Present State of Ohio - 004465 CERTIFICATIONS Board Certified Sports Clinical Specialist (SCS) 2016-Present American Physical Therapy Association Certified in Dry Needling (Cert. DN) 2015-Present Spinal Manipulation Institute Certified Clinical Instructor (CCI) 2014-Present American Physical Therapy Association Basic Life Support for Healthcare Providers 2016-Present American Heart Association Certified Strength and Conditioning Specialist (CSCS) 2013-Present National Strength and Conditioning Association Certified Athletic Trainer (ATC) 2011-Present National Athletic Training Association Board of Certification Certification number: 2000005566 EXPERIENCE The Ohio State University, Columbus, OH 2016-Present The Ohio State University Sports Medicine Physical Therapist/Athletic Training Outreach Services The Ohio State University, Columbus, OH 2014-2015 The Ohio State University Sports Medicine Sports Physical Therapy Resident Matthew P. Brancaleone, PT, DPT, SCS, AT, CSCS [email protected] USA Deaf Sports Federation Summer 2013 2013 Summer Deaflympics Games in Sofia, Bulgaria Athletic Trainer Gallaudet University, Washington D.C. -

Congressional Record United States Th of America PROCEEDINGS and DEBATES of the 113 CONGRESS, FIRST SESSION
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 113 CONGRESS, FIRST SESSION Vol. 159 WASHINGTON, TUESDAY, JULY 16, 2013 No. 101 House of Representatives The House met at noon and was tember, we will be only 2 days away Instead of learning the details of the called to order by the Speaker pro tem- from the 1-year anniversary of the attack and the U.S. response in public pore (Mr. BENTIVOLIO). Benghazi attacks. This looming anni- hearings, the American people may in- f versary should stand as a stark re- stead read about it in one of the books minder of the many unanswered ques- that have been announced in recent DESIGNATION OF SPEAKER PRO tions that remain about what actually weeks. It is clear that the survivors TEMPORE happened that night and how the ad- from the consulate and the annex have The SPEAKER pro tempore laid be- ministration chose to respond or not worked with authors on two separate fore the House the following commu- respond to the Americans under as- books that are scheduled to be pub- nication from the Speaker: sault during that 8-hour period. lished over the next year. WASHINGTON, DC, That is why, over the next 3 weeks, I The first, ‘‘Under Fire: The Untold July 16, 2013. will be coming to the floor regularly to Story of the Attack in Benghazi,’’ de- I hereby appoint the Honorable KERRY remind the American people about the scribes in vivid, minute-by-minute de- BENTIVOLIO to act as Speaker pro tempore on key questions that remain to be an- tail the assault on the U.S. -

Of 6 2013 Deaflympics Swimming Technical Regulations 1. EVENTS
2013 Deaflympics Swimming Technical Regulations 1. EVENTS The competitions will be conducted from 27 July 2013 to 2 August 2013. The swimming competitions will consist of the following 38 events (19 for men and 19 for women). Individual events: Freestyle - 50m, 100m, 200m, 400m, 800m (women only), & 1500m (men only) Backstroke - 50m, 100m, 200m Breaststroke - 50m, 100m, 200m Butterfly - 50m, 100m, 200m Individual Medley - 200m, 400m Team relay events: Freestyle - 4x100m, 4x200m Medley – 4x100m 2. PARTICIPANTS 2.1. Age Eligibility There are no age restrictions. 2.2. Number of Swimmers Each National Federation may enter a maximum number of 26 men and 26 women swimmers. Each National Federation may enter three (3) competitors in each individual event and one (1) team in each relay event. Swimmers entered for individual events and for the relays may act as reserves for the relays. The composition of teams for the relay events must be announced at least one hour before the corresponding sessions begin. Team compositions may be changed between the heats and the final. When preliminaries are swum, medals shall be awarded to those swimmers who swam in the preliminaries and the final race. 2.3. Qualification Times ICSD has established standard qualification times for each individual and relay event at the Deaflympics. See additional documents for Qualification Times. The qualification times must be obtained in the period from 01 July 2011 to 01 July 2013. All National Federation having no swimmers qualified may enter one man and one woman regardless of time standards in competitions of their choice with evidence of participation in national and international competition. -

Coach Resource Guide
1 TABLE OF CONTENTS PREFACE .......................................................................................... 3 ACKNOWLEDGEMENTS........................................................................ 5 HEARING LOSS .................................................................................. 6 COMMUNICATION ............................................................................. 15 MEETING LANGUAGE AND SKILL NEEDS ................................................ 19 CREATING A D/HH-FRIENDLY ENVIRONMENT ......................................... 21 SPECIFIC SPORT ADAPTATIONS .......................................................... 26 WORKING WITH OFFICIALS ................................................................. 28 PATHWAY FOR MANITOBA’S D/HH ATHLETES IN ELITE DEAF SPORTS .......... 30 OTHER SPORT SPECIFIC RESOURCES .................................................. 30 BIBLIOGRAPHY ................................................................................ 31 2 2 PREFACE People who are deaf and hard of hearing (D/HH) are said to have an “invisible” disability. There is no wheelchair or white cane; no unusual gait or prosthetic. Hearing aids are becoming so small as to be invisible and with the advent of cochlear implants, many deaf and hard of hearing children speak with ease. While it is hard to see, being D/HH has a profound impact on the development of a child and will have a life-long affect as they move through the hearing world. It is often difficult for the hearing to understand exactly what it is -

2013 年索菲亞第22 屆聽障達福林匹克運動會SOFIA 2013 22Th SUMMER
2013 年索菲亞第 22 屆聽障 達福林匹克運動會 SOFIA 2013 22th SUMMER DEAFYMPICS 跆拳道隊打項目返國報告書 報告人 邱共鉦 127 壹、前言 7月22日(一)跆拳道隊在丁主任的帶領下,搭乘國泰航空(CX701)19時20由桃園中正機場 (TPE)第一航站出發,行 程 1小時45分,先 抵達香港國際機場(HKG)再轉乘土耳其航空(TK71), 7月22日(一)23時25分往土耳其出發,航程11時20分。7月23日(二)5時45分抵達伊斯坦堡國 際航站(IST),再搭乘土耳其航空7月23日(二)7時30分出發,往保加利亞首都索菲亞,航程1 小時15分,於7月23日(二)8時45分平安抵達索菲亞國際航站(SOF)。 前二日並無賽程,除了調整時差外,也到比賽場地練習。技術會議時間多次修正,並未 能及時通知,競賽表列名單也所更動。就連小小的一張對戰表也吝嗇提供,如此服務態度, 令人傻眼。比賽場館亦無冷氣設備,所有人員全都汗流浹背。整體而言,比賽辦得七零八落, 絲毫沒有國際水準。 對打項目獲得女子-49Kg級銀牌(彭思庭)、女子-57Kg級銅牌呂思柔。品試項目女子彭思 庭獲得銀牌,男女配對獲得銅牌(林何勳、彭思庭),彭思庭獨獲2銀1銅成為跆拳道比賽場的 大贏家,而男子出賽皆墨,無緣獲得獎牌。 全隊於07月31日(三)於18時赴索菲亞(SOF)第二航站機場辦理登機手續時,因警方研判 有爆裂物,警方將旅客疏散至戶外,而耽擱一些時間。協會湯副秘書長及王秘書全程陪同協 助,在機場外苦等,令人感動。我隊於07月31日(三)23時10分抵達伊斯坦堡國際航站(IST), 再轉土耳其航空(TK70)08月01日(四)0時55分出發,航程10時25分,於08月01日(四)16時20 分抵達香港(HKG),再轉港龍航空(KA482)08月01日(四)18時30分出發,於8月01日(四)20時 15分平安返抵國門。 在機場受到民眾熱情迎接,此行雖未能奪金,但也替國家盡一份心力,感到榮耀。感謝 教育部體育署及協會長官居中安排赴賽,使我國聽障選手有機會參與國際盛會,並贏得友誼 及尊重。 貳、參賽前的準備 當我們抵達索菲亞(SOFIA)時,韓國隊也造訪。在機場見到韓國隊大軍抵達索菲亞,同 時有韓國民眾或政府官員安排接機。浩大的聲勢似乎影響到團員壓力,因此我意有所指說, 韓國跆拳道選手偷指誰是林伯聰的事情(韓籍教練問韓國選手誰是林伯聰),告訴選手我們也 很強,也造成韓國隊的壓力。並說中華跆拳道也在強隊之林,連韓國選手也怕我們三分,到 索菲亞就是打,專心應戰發揮應有水準即可。 在抵達METROPOLITAN飯店後,稍加梳洗後,11時就地在飯店附近廣場簡單練習,選手的 踢擊表現都正常。當日並要求選手不要午睡,裨益調整時差。21時再赴飯店停車場練習50分 鐘後結束後落幕,得以卸下疲累,好好梳洗及休息。由於氣候比台灣乾燥,謝承霖有流鼻血 現象,已經屬咐他戴口罩,以保持鼻部空氣濕潤。 7月24日(三) 本隊1000時練習,1600練習;彭思庭上午練習體重已將控制在48.5Kg,但為了保險起見, 我仍建議彭思庭在通過大會磅秤的試磅確認後,才可以多吃一點,而彭思庭亦認為如此。 2000團務會議重點摘要: 1. 比賽時,選手不得攜帶助聽器,違者取消參賽資格。 2. 本隊隔日可赴練習場練習,時間為0800-1000整。 128 3. 每一位皆需要接受藥檢。 4. 飯店用膳時間為:早餐0630-0900、中餐1200-1500、晚餐1900-2200。 5. 比賽期間仍希望回飯店用膳。 6. 水無限供應,不足時可領取。 7. 本次選手參與選手約為2000餘人,其中以俄羅斯派出500餘人最多,其獲得金牌者, -

Extensions of Remarks E1061 EXTENSIONS of REMARKS
July 16, 2013 CONGRESSIONAL RECORD — Extensions of Remarks E1061 EXTENSIONS OF REMARKS HONORING HENRY POSEY 2007, Father Pat was also assigned Pastor of assignment without the distraction of hunger, Saint Mark Catholic Church in Gary. Through- guarantees healthy meals to struggling fami- HON. STEPHEN LEE FINCHER out the years, Father Gaza has served in var- lies who have been hit hard by the recent eco- OF TENNESSEE ious organizations throughout Northwest Indi- nomic downturn, and lends to the economic ana and Gary, including the LaPorte County advancement of communities across the coun- IN THE HOUSE OF REPRESENTATIVES FEMA Food Program, the Gary Ten Point Co- try. Tuesday, July 16, 2013 alition, the Gary Urban Enterprise Association, Forty-seven million people experience food Mr. FINCHER. Mr. Speaker, I rise today to the Gary branch of the NAACP, Rebuilding insecurity in the United States. In New York honor the retirement of Henry Posey from the Together, and the Catholic Youth Organiza- alone, over three million New Yorkers receive Memphis Fire Department. tion. Father Gaza’s involvement with these or- food stamps. This bill as it currently stands is Mr. Posey has devoted his life to a career ganizations evidences his absolute commit- an attack on the nutrition programs, specifi- of public service. As a fire fighter and the retir- ment to minister and tirelessly work and advo- cally food stamps. ing Division Chief for the Memphis Fire De- cate on behalf of ‘‘the least amongst us.’’ We have an unparalleled moment of oppor- partment, he worked to keep communities in Those who are without, those who suffer tunity to generate policy that is in tune with the the Eighth District of Tennessee safe for over physically or are challenged physiologically, circumstances of ALL of the American peo- 36 years. -
![Barbara Duschet [Barbara.Duschet@Oegsv.At] Gesendet: Donnerstag, 14](https://docslib.b-cdn.net/cover/7752/barbara-duschet-barbara-duschet-oegsv-at-gesendet-donnerstag-14-4927752.webp)
Barbara Duschet [[email protected]] Gesendet: Donnerstag, 14
Von: Barbara Duschet [[email protected]] Gesendet: Donnerstag, 14. Oktober 2010 15:06 An: [email protected] Betreff: WG: Minimum Age ‐ 2013 Summer Deaflympics Wichtigkeit: Hoch Liebe Mitglieder! Mit diesem Email möchte der ICSD Vorstand Euch informieren, dass das Mindestalter nur für die Sommer Deaflympics genehmigt wurde. Vor kurzem in Taipei 2009, gab es für einige Sportarten keine Mindestaltersbegrenzung. Die Bezeichnung in den Technischen Vorschriften war wie folgt: 4.1. Altersbegrenzung Es gibt keine Altersbegrenzung. Obenstehendes bedeutet, dass jeder der 8 Jahre alt ist, bei den Sommer Deaflympics mitmachen darf, was aber kein gutes Bild für diese Organisation macht, speziell im Vordergrund nicht vor dem IOC. Das ist nicht gut! Der Vorstand bestätigt, dass das Mindestalter für alle Sportarten 14 Jahre alt ist. Trotzdem, auch wenn bei einer Sportart bereits höher als 14 Jahren angegeben ist, wie auch entsprechend den Olympischen Spielen. Hierfür wird es auf das höhere Alter raufgesetzt und angepasst. Auch das Geburtsjahr muss stattdessen zum Einsatz kommen, nicht der Stichtag der Eröffnungszeremonie oder dem Tag des ersten Wettkamfes. Untenstehende Disziplinen werden ab den 2013 Sommer Deaflympics gültig: Badminton Basketball Beach Volleyball Bowling (12 Jahre akzeptieren wir nicht als Mindestalter. Es wurde erhöht auf 14) Fußball (14, 15, & 16 Jährige benötigen das Elterliche Einverständnis) Orientierungslauf Schießen Schwimmen Tischetennis Volleyball All die anderen Disziplinen, welche oben nicht aufgelistet sind, werden nicht geändert und folgen dem Alter 14 oder älter entsprechend dem, was in Taipei 2009 festgesetzt wurde. Nochmal, das Mindestalter 14 ist nur für die Sommer Deaflympics. Das Mindestalter für die Sommer Deaflympics 2013 können Sie auf unserer Homepage einsehen - http://www.deaflympics.com/pdf/Age-Eligibility-All-Sports.pdf Die technischen Vorschriften für den Sommersport werden später aktualisiert. -

Ritreportervol59no13.Pdf
12 18 09 | reportermag.com TIGERS BATTLE THE BOMBERS RIT Wrestling takes on Ithaca ADDICTION IN RELATIONSHIPS Porn, smokes and booze WITR CANCELS 6 SHOWS Summer block buster preview EDITOR’S NOTE TABLE OF CONTENTS 12 18 09 | VOLUME 59 | ISSUE 13 EDITOR IN CHIEF Andy Rees | [email protected] SINGULARITY MANAGING EDITOR Madeleine Villavicencio Whoever decided that spring is the season of love was kidding himself. Sure, the flowers are blooming and | [email protected] there’s birds singing, but symbolism aside, there’s not much going on there. COPY EDITOR Michael Conti Winter, on the other hand, is really when love is in the air. The nights are cold, you’ve let yourself go a | [email protected] little after Thanksgiving, and you’re feeling a little vulnerable because Grandma asked you why you never NEWS EDITOR Emily Bogle bring anyone to Christmas dinner. It’s the perfect time of year to wind up in a relationship. | [email protected] If you take a look at the distribution of birthdays throughout a year, the numbers peak between August LEISURE EDITOR John Howard and November. Those months are conveniently three trimesters away from the prior winter. Let’s face it, if | [email protected] you’re a fall baby, you might be the result of “the house is cold, let’s keep warm” love making. FEATURES EDITOR Michael Barbato In any case, Winter is a dangerous time to be single. The bars are packed with people in ugly sweaters | [email protected] looking for some light conversation and a little heavy breathing. -

Age Eligibility for All Sports 2013 Summer Deaflympics
Age Eligibility for all Sports 2013 Summer Deaflympics Athletics 4.1. Age Eligibility Junior Athletes Any athlete aged 18 or 19 years on 31 December 2013 (born in 1994/1995) may compete in any event except the Marathon. Youth Athletes Any athlete aged 16 or 17 years on 31 December 2013 (born in 1996/1997) may compete in any event except the throwing events (male athletes), Decathlon, 10,000m and Marathon events. Athletes Younger than 16 No athlete younger than 16 years of age on 31 December 2013 (born in 1998 or after) may be entered. Badminton 4.1. Age Eligibility All badminton competitors must be at least 14 years old in 2013 (born in 1999 or before). Basketball 4.1. Age Eligibility All basketball competitors must be at least 14 years old in 2013 (born in 1999 or before). Beach Volleyball 4.1. Age Eligibility All beach volleyball competitors must be at least 14 years old in 2013 (born in 1999 or before). Bowling 4.1. Age Eligibility All tenpin bowling competitors must be at least 14 years old in 2013 (born in 1999 or before). Cycling Road 4.1. Age Eligibility All road cyclist competitors must be at least 17 years old in 2013 (born in 1996 or before). Football 4.1. Age Eligibility All football competitors must be at least 14 years old in 2013 (born in 1999 or before). The National Federation must obtain parental authorization for athletes 16 years and under (born in 1997/1998/1999). Judo 4.1. Age Eligibility All judo competitors must be at least 17 years old in 2013 (born in 1996 or before).