Exploring the Atom, 1919-1932
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rutherford's Nuclear World: the Story of the Discovery of the Nuc
Rutherford's Nuclear World: The Story of the Discovery of the Nuc... http://www.aip.org/history/exhibits/rutherford/sections/atop-physic... HOME SECTIONS CREDITS EXHIBIT HALL ABOUT US rutherford's explore the atom learn more more history of learn about aip's nuclear world with rutherford about this site physics exhibits history programs Atop the Physics Wave ShareShareShareShareShareMore 9 RUTHERFORD BACK IN CAMBRIDGE, 1919–1937 Sections ← Prev 1 2 3 4 5 Next → In 1962, John Cockcroft (1897–1967) reflected back on the “Miraculous Year” ( Annus mirabilis ) of 1932 in the Cavendish Laboratory: “One month it was the neutron, another month the transmutation of the light elements; in another the creation of radiation of matter in the form of pairs of positive and negative electrons was made visible to us by Professor Blackett's cloud chamber, with its tracks curled some to the left and some to the right by powerful magnetic fields.” Rutherford reigned over the Cavendish Lab from 1919 until his death in 1937. The Cavendish Lab in the 1920s and 30s is often cited as the beginning of modern “big science.” Dozens of researchers worked in teams on interrelated problems. Yet much of the work there used simple, inexpensive devices — the sort of thing Rutherford is famous for. And the lab had many competitors: in Paris, Berlin, and even in the U.S. Rutherford became Cavendish Professor and director of the Cavendish Laboratory in 1919, following the It is tempting to simplify a complicated story. Rutherford directed the Cavendish Lab footsteps of J.J. Thomson. Rutherford died in 1937, having led a first wave of discovery of the atom. -

The Development of Military Nuclear Strategy And
The Development of Military Nuclear Strategy and Anglo-American Relations, 1939 – 1958 Submitted by: Geoffrey Charles Mallett Skinner to the University of Exeter as a thesis for the degree of Doctor of Philosophy in History, July 2018 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. (Signature) ……………………………………………………………………………… 1 Abstract There was no special governmental partnership between Britain and America during the Second World War in atomic affairs. A recalibration is required that updates and amends the existing historiography in this respect. The wartime atomic relations of those countries were cooperative at the level of science and resources, but rarely that of the state. As soon as it became apparent that fission weaponry would be the main basis of future military power, America decided to gain exclusive control over the weapon. Britain could not replicate American resources and no assistance was offered to it by its conventional ally. America then created its own, closed, nuclear system and well before the 1946 Atomic Energy Act, the event which is typically seen by historians as the explanation of the fracturing of wartime atomic relations. Immediately after 1945 there was insufficient systemic force to create change in the consistent American policy of atomic monopoly. As fusion bombs introduced a new magnitude of risk, and as the nuclear world expanded and deepened, the systemic pressures grew. -

Patrick Blackett: Sailor, Scientist, Socialist, Chris Eldridge
Naval War College Review Volume 57 Article 31 Number 1 Winter 2004 Patrick Blackett: Sailor, Scientist, Socialist, Chris Eldridge Follow this and additional works at: https://digital-commons.usnwc.edu/nwc-review Recommended Citation Eldridge, Chris (2004) "Patrick Blackett: Sailor, Scientist, Socialist,," Naval War College Review: Vol. 57 : No. 1 , Article 31. Available at: https://digital-commons.usnwc.edu/nwc-review/vol57/iss1/31 This Book Review is brought to you for free and open access by the Journals at U.S. Naval War College Digital Commons. It has been accepted for inclusion in Naval War College Review by an authorized editor of U.S. Naval War College Digital Commons. For more information, please contact [email protected]. 156 NAVAL WAR COLLEGE REVIEW Eldridge: Patrick Blackett: Sailor, Scientist, Socialist, understanding with the Soviet Union (a he was the heart and soul of the Cold position favored by some influential War military-academic-industrial com- opinions in the United States) nor to cre- plex. In this book, sixteen authors at- ate trouble for the French government, tempt to shed light on Blackett’s role in seemingly both dependent on and threat- that story. The collection includes pa- ened by the French Communist Party. pers presented at a 1998 conference The Soviet-menace card was played on commemorating Blackett at Cambridge several occasions in the unfolding de- University, as well as other recent writ- bate, but in general it was subordinated ings about him. to more abstract arguments of enlight- Not surprisingly, the compendium of- ened self-interest. Moreover, it was fers a range of perspectives on events clear to many in the administration that and issues with which Blackett was as- too great an emphasis on the immi- sociated, rather than a comprehensive nence of war with Russia would scuttle examination of his life and work. -

Chem 103, Section F0F Unit I
Lecture 4 - Observations that Led to the Chem 103, Section F0F Nuclear Model of the Atom Unit I - An Overview of Chemistry Dalton’s theory proposed that atoms were indivisible particles. Lecture 4 • By the late 19th century, this aspect of Dalton’s theory was being challenged. • Work with electricity lead to the discovery of the electron, • Some observations that led to the nuclear model as a particle that carried a negative charge. for the structure of the atom • The modern view of the atomic structure and the elements • Arranging the elements into a (periodic) table 2 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom The cathode ray In 1897, J.J. Thomson (1856-1940) studies how cathode rays • Cathode rays were shown to be electrons are affected by electric and magnetic fields • This allowed him to determine the mass/charge ration of an electron Cathode rays are released by metals at the cathode 3 4 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom In 1897, J.J. Thomson (1856-1940) studies how cathode rays In 1897, J.J. Thomson (1856-1940) studies how cathode rays are affected by electric and magnetic fields are affected by electric and magnetic fields • Thomson estimated that the mass of an electron was less • Thomson received the 1906 Nobel Prize in Physics for his that 1/1000 the mass of the lightest atom, hydrogen!! work. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -
![Arxiv:1211.4061V3 [Physics.Hist-Ph] 8 Feb 2013](https://docslib.b-cdn.net/cover/9997/arxiv-1211-4061v3-physics-hist-ph-8-feb-2013-949997.webp)
Arxiv:1211.4061V3 [Physics.Hist-Ph] 8 Feb 2013
From cosmic ray physics to cosmic ray astronomy: Bruno Rossi and the opening of new windows on the universe Luisa Bonolis Via Cavalese 13 – 00135 Rome, Italy [email protected] Abstract Bruno Rossi is considered one of the fathers of modern physics, being also a pioneer in virtually every aspect of what is today called high-energy astrophysics. At the beginning of 1930s he was the pioneer of cosmic ray research in Italy, and, as one of the leading actors in the study of the nature and behavior of the cosmic radiation, he witnessed the birth of particle physics and was one of the main investigators in this fields for many years. While cosmic ray physics moved more and more towards astrophysics, Rossi continued to be one of the inspirers of this line of research. When outer space became a reality, he did not hesitate to leap into this new scientific dimension. Rossi’s intuition on the importance of exploiting new technological windows to look at the universe with new eyes, is a fundamental key to understand the profound unity which guided his scientific research path up to its culminating moments at the beginning of 1960s, when his group at MIT performed the first in situ measurements of the density, speed and direction of the solar wind at the boundary of Earth’s magnetosphere, and when he promoted the search for extra-solar sources of X rays. A visionary idea which eventually led to the breakthrough experiment which discovered Scorpius X-1 in 1962, and inaugurated X-ray astronomy. -

Historical Background Prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (Retired) University of New Brunswick
1 Historical Background prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (retired) University of New Brunswick Summary: A review of the historical background for the development of nuclear energy is given to set the scene for the discussion of CANDU reactors. Table of Contents 1 Growth of Science and Technology......................................................................................... 2 2 Renowned Scientists............................................................................................................... 4 3 Significant Achievements........................................................................................................ 6 3.1 Niels Bohr........................................................................................................................ 6 3.2 James Chadwick .............................................................................................................. 6 3.3 Enrico Fermi .................................................................................................................... 6 4 Nuclear Fission........................................................................................................................ 7 5 Nuclear Energy........................................................................................................................ 7 6 Acknowledgments................................................................................................................... 8 List of Figures Figure 1 Timeline of significant discoveries -

Absolute Zero, Absolute Temperature. Absolute Zero Is the Lowest
Contents Radioactivity: The First Puzzles................................................ 1 The “Uranic Rays” of Henri Becquerel .......................................... 1 The Discovery ............................................................... 2 Is It Really Phosphorescence? .............................................. 4 What Is the Nature of the Radiation?....................................... 5 A Limited Impact on Scientists and the Public ............................ 6 Why 1896? .................................................................. 7 Was Radioactivity Discovered by Chance? ................................ 7 Polonium and Radium............................................................. 9 Marya Skłodowska .......................................................... 9 Pierre Curie .................................................................. 10 Polonium and Radium: Pierre and Marie Curie Invent Radiochemistry.. 11 Enigmas...................................................................... 14 Emanation from Thorium ......................................................... 17 Ernest Rutherford ........................................................... 17 Rutherford Studies Radioactivity: ˛-and ˇ-Rays.......................... 18 ˇ-Rays Are Electrons ....................................................... 19 Rutherford in Montreal: The Radiation of Thorium, the Exponential Decrease........................................... 19 “Induced” and “Excited” Radioactivity .................................... 20 Elster -
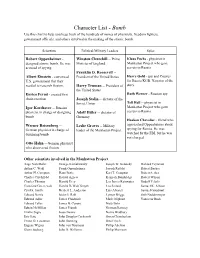
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Commentary & Notes Hans Bethe
J. Natn.Sci.Foundation Sri Lanka 2005 33(1): 57-58 COMMENTARY & NOTES HANS BETHE (1906-2005) Hans Albrecht Bethe who died on 6 March 2005 within the sun is hydrogen which is available in at the age of 98, was one of the most influential tremendous volume. Near the centre of the sun, and innovative theoretical physicists of our time. hydrogen nuclei can be squeezed under enormous pressure to become the element helium. If the Bethe was born in Strasbourg, Alsace- mass of hydrogen nucleus can be written as 1, Lorraine (then part of Germany) on 2 July 1906. Bethe showed that each time four nuclei of His father was a University physiologist and his hydrogen fused together as helium, their sum was mother Jewish, the daughter of a Strasbourg not equal to 4. The helium nucleus weighs about professor of Medicine. Bethe moved to Kiel and 0.7% less, or just 3.993 units of weight. It is this Frankfurt from where he went to Munich to carry missing 0.7% that comes out as a burst of explosive out research in theoretical physics under Arnold energy. The sun is so powerful that it pumps 4 Sommerfeld, who was not only a great teacher, million tons of hydrogen into pure energy every but an inspiring research supervisor as well. second. Einstein's famous equation, E = mc2, Bethe received his doctorate in 1928, for the work provides a clue to the massive energy that reaches he did on electron diffraction. He then went to the earth from the sun, when 4 million tons of the Cavendish laboratory in Cambridge on a hydrogen are multiplied by the figure c2 (square Rockefeller Fellowship, and Rome, before of the speed of light). -

Patrick Blackett | Atomic Heritage Foundation
7/19/2018 Patrick Blackett | Atomic Heritage Foundation MENU STORE DONATE Patrick Blackett Physicist, United Kingdom Born Nov 18 1897 Scientist, Nobel Prize Winner Patrick Blackett (1897-1974) was an accomplished British scientist who won the Nobel Prize in Physics in 1948. EARLY YEARS Patrick Maynard Stuart Blackett was born in Kensington, London on November 18th, 1897. He entered the Osborne Royal Naval College in 1910, and transferred to the Dartmouth Royal Naval College two years later. Before he earned his degree, though, World War I broke out, and Blackett was sent off to fight. He saw combat at the Battle of Falkland Islands in 1914 and the Battle of Jutland in 1916, emerging from the war as a lieutenant. When the war concluded, the British Admiralty sent Blackett to Cambridge to complete his studies. Enjoying his studies at Cambridge immensely, Blackett decided to resign from the Royal Navy and focus solely upon studying mathematics and sub-atomic physics at Cambridge. Two years later, he completed his undergraduate degree and stayed on at the university as a Bye-Fellow studying under Ernest Rutherford at the Cavendish Laboratory. After completing this fellowship, Blackett became a fellow at King’s College, where he would remain until 1933. While conducting research at Cambridge, it is believed that a young, distraught graduate student named J. Robert Oppenheimer attempted to poison Blackett with an apple laced with toxic chemicals. Blackett was Oppenheimber’s head tutor at the time, and Oppenheimer found Blackett to be brilliant but also extremely demanding. Blackett insisted that Oppenheimer spend more time doing lab work while Oppenheimer believed his time and talents should be devoted to theoretical physics. -

A Layman's Guide to M-Theory1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CERN Document Server CTP-TAMU-23/98 hep-th/9805177 A Layman’s Guide to M-Theory1. M. J. Duff Center for Theoretical Physics Texas A&M University, College Station, Texas 77843 ABSTRACT The best candidate for a fundamental unified theory of all physical phenomena is no longer ten-dimensional superstring theory but rather eleven-dimensional M-theory. In the words of Fields medalist Edward Witten, “M stands for ‘Magical’, ‘Mystery’ or ‘Membrane’, according to taste”. New evidence in favor of this theory is appearing daily on the internet and represents the most exciting development in the subject since 1984 when the superstring revolution first burst on the scene. 1Talk delivered at the Abdus Salam Memorial Meeting, ICTP, Trieste, November 1997. 1 Abdus Salam The death of Abdus Salam was a great loss not only to his family and to the physics community; it was a loss to all mankind. For he was not only one of the finest physicists of the twentieth century, having unified two of the four fundamental forces in Nature, but he also dedicated his life to the betterment of science and education in the Third World and to the cause of world peace. Although he won the Nobel Prize for physics, a Nobel Peace Prize would have been entirely appropriate. At the behest of Patrick Blackett, Salam moved to Imperial College, London, in 1957 where he founded the Theoretical Physics Group. He remained at Imperial as Profesor of Physics for the rest of his carreer.