Towards Limiting QT Interval Prolongation and Arrhythmia Risk in Citalopram Use Yihong Zhang1, Adrian Baranchuk2, Jules C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

Use of Human Plasma Samples to Identify Circulating Drug Metabolites That Inhibit Cytochrome P450 Enzymes
1521-009X/44/8/1217–1228$25.00 http://dx.doi.org/10.1124/dmd.116.071084 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 44:1217–1228, August 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Use of Human Plasma Samples to Identify Circulating Drug Metabolites that Inhibit Cytochrome P450 Enzymes Heather Eng and R. Scott Obach Pfizer Inc., Groton, Connecticut Received April 19, 2016; accepted June 3, 2016 ABSTRACT Drug interactions elicited through inhibition of cytochrome P450 fractions were tested for inhibition of six human P450 enzyme (P450) enzymes are important in pharmacotherapy. Recently, activities (CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and greater attention has been focused on not only parent drugs CYP3A4). Observation of inhibition in fractions that correspond to inhibiting P450 enzymes but also on possible inhibition of these the retention times of metabolites indicates that the metabolite Downloaded from enzymes by circulating metabolites. In this report, an ex vivo method has the potential to contribute to P450 inhibition in vivo. Using whereby the potential for circulating metabolites to be inhibitors of this approach, norfluoxetine, hydroxyitraconazole, desmethyldiltia- P450 enzymes is described. To test this method, seven drugs and zem, desacetyldiltiazem, desethylamiodarone, hydroxybupropion, their known plasma metabolites were added to control human erythro-dihydrobupropion, and threo-dihydrobupropion were iden- plasma at concentrations previously reported to occur in humans -

Aerobic Treatment of Selective Serotonin Reuptake Inhibitors in Landfill Leachate Ove Bergersen1*, Kine Østnes Hanssen2 and Terje Vasskog2,3
Bergersen et al. Environmental Sciences Europe (2015) 27:6 DOI 10.1186/s12302-014-0035-0 RESEARCH Open Access Aerobic treatment of selective serotonin reuptake inhibitors in landfill leachate Ove Bergersen1*, Kine Østnes Hanssen2 and Terje Vasskog2,3 Abstract Background: Pharmaceuticals used in human medical care are not completely eliminated in the human body and can enter the municipal sewage sludge system and leachate water from landfill both as the parent compound and as their biologically active metabolites. The selective serotonin reuptake inhibitors (SSRIs) have a large potential for unwanted effects on nontarget organisms in the environment. Leachates from active or old closed landfills are often treated with continuous stirring and simple aeration in a pond/lagoon before infiltration into the environment. The aim of this work was to simulate the reduction of five SSRIs (citalopram, fluoxetine, paroxetine, sertraline and fluvoxamine) and three of their metabolites (desmethylcitalopram, didesmethylcitalopram and norfluoxetine) during aerobic treatment of leachate from landfills. This landfill leachate-simulation experiment was performed to see what happens with the pharmaceuticals during aerated treatment and continuous stirring of landfill leachate for 120 h. It is important to establish whether different pollutants such as pharmaceuticals can be removed (oxidized or otherwise degraded) or not before infiltration into the environment. Results: All the SSRIs had a significant concentration reduction during the aeration treatment process. Total SSRI concentrations were reduced significantly during aerobic treatment, and the individual SSRIs were reduced by 89% to 100% after 120 h. Among the high-concentration samples, fluoxetine (10 mg L−1) was the least degraded with 93% concentration reduction. -

Determination of Antidepressants in Human Plasma by Modified Cloud
pharmaceuticals Article Determination of Antidepressants in Human Plasma by Modified Cloud-Point Extraction Coupled with Mass Spectrometry El˙zbietaGniazdowska 1,2 , Natalia Korytowska 3 , Grzegorz Kłudka 3 and Joanna Giebułtowicz 3,* 1 Łukasiewicz Research Network, Industrial Chemistry Institute, 8 Rydygiera, 01-793 Warsaw, Poland; [email protected] 2 Department of Bioanalysis and Drugs Analysis, Doctoral School, Medical University of Warsaw, 61 Zwirki˙ i Wigury, 02-091 Warsaw, Poland 3 Department of Bioanalysis and Drugs Analysis, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha, 02-097 Warsaw, Poland; [email protected] (N.K.); [email protected] (G.K.) * Correspondence: [email protected] Received: 5 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Cloud-point extraction (CPE) is rarely combined with liquid chromatography coupled to mass spectrometry (LC–MS) in drug determination due to the matrix effect (ME). However, we have recently shown that ME is not a limiting factor in CPE. Low extraction efficiency may be improved by salt addition, but none of the salts used in CPE are suitable for LC–MS. It is the first time that the influences of a volatile salt—ammonium acetate (AA)—on the CPE extraction efficiency and ME have been studied. Our modification of CPE included also the use of ethanol instead of acetonitrile to reduce the sample viscosity and make the method more environmentally friendly. We developed and validated CPE–LC–MS for the simultaneous determination of 21 antidepressants in plasma that can be useful for clinical and forensic toxicology. The selected parameters included Triton X-114 concentration (1.5 and 6%, w/v), concentration of AA (0, 10, 20 and 30%, w/v), and pH (3.5, 6.8 and 10.2). -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Pharmacokinetic and Pharmacodynamic Interactions Between Antiepileptics and Antidepressants Domenico Italiano University of Messina, Italy
University of Kentucky UKnowledge Psychiatry Faculty Publications Psychiatry 11-2014 Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants Domenico Italiano University of Messina, Italy Edoardo Spina University of Messina, Italy Jose de Leon University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/psychiatry_facpub Part of the Psychiatry and Psychology Commons Repository Citation Italiano, Domenico; Spina, Edoardo; and de Leon, Jose, "Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants" (2014). Psychiatry Faculty Publications. 40. https://uknowledge.uky.edu/psychiatry_facpub/40 This Article is brought to you for free and open access by the Psychiatry at UKnowledge. It has been accepted for inclusion in Psychiatry Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants Notes/Citation Information Published in Expert Opinion on Drug Metabolism & Toxicology, v. 10, Issue 11, p. 1457-1489. © 2014 Taylor & Francis Group This is an Accepted Manuscript of an article published by Taylor & Francis Group in Expert Opinion on Drug Metabolism & Toxicology in Nov. 2014, available online: http://www.tandfonline.com/10.1517/ 17425255.2014.956081 Digital Object Identifier (DOI) http://dx.doi.org/10.1517/17425255.2014.956081 This article is available at UKnowledge: https://uknowledge.uky.edu/psychiatry_facpub/40 1 This is an Accepted Manuscript of an article published by Taylor & Francis Group in Expert Opinion on Drug Metabolism & Toxicology in Nov. -

And S-Citalopram and Desmethylcitalopram in Alzheimer's
J Pharmacokinet Pharmacodyn (2016) 43:99–109 DOI 10.1007/s10928-015-9457-6 ORIGINAL PAPER A population pharmacokinetic model for R- and S-citalopram and desmethylcitalopram in Alzheimer’s disease patients with agitation 1 1,12 2 3 Ayman Akil • Robert R. Bies • Bruce G. Pollock • Dimitrios Avramopoulos • 4 5 6 7 D. P. Devanand • Jacobo E. Mintzer • Anton P. Porsteinsson • Lon S. Schneider • 8 9 10 11 Daniel Weintraub • Jerome Yesavage • David M. Shade • Constantine G. Lyketsos Received: 30 June 2015 / Accepted: 14 November 2015 / Published online: 26 November 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract The citalopram for Alzheimer’s disease trial any patient-specific covariates, the population estimate of evaluated citalopram for the management for agitation in the metabolic clearance of citalopram was 8.6 (R-citalo- Alzheimer’s disease patients. Sparse data was available pram) and 14 L/h (S-citalopram). The population estimate from this elderly patient population. A nonlinear mixed of the clearance of desmethylcitalopram was 23.8 (R-Dcit) effects population pharmacokinetic modeling approach was and 38.5 L/h (S-Dcit). Several patient-specific covariates used to describe the pharmacokinetics of R- and S-citalo- were found to have a significant effect on the pharma- pram and their primary metabolite (desmethylcitalopram). cokinetics of R,S-citalopram and desmethylcitalopram. A A structural model with 4 compartments (one compart- significant difference in the metabolic clearance of R-ci- ment/compound) with linear oral absorption and elimina- talopram between males and females (13 vs 9.05 L/h) was tion described the data adequately. -

In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus Carpio)
molecules Communication In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio) Viktoriia Burkina 1,2,* , Sidika Sakalli 1, Pham Thai Giang 1,3, KateˇrinaGrabicová 1 , Andrea Vojs Sta ˇnová 1,4, Galia Zamaratskaia 1,2 and Vladimir Zlabek 1 1 South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Faculty of Fisheries and Protection of Waters, University of South Bohemia in Ceske Budejovice, Zatisi 728/II, 389 25 Vodˇnany, Czech Republic; [email protected] (S.S.); [email protected] (P.T.G.); [email protected] (K.G.); [email protected] (A.V.S.); [email protected] (G.Z.); [email protected] (V.Z.) 2 Department of Molecular Sciences, Swedish University of Agricultural Sciences, P.O. Box 7015, SE-750 07 Uppsala, Sweden 3 Research Institute for Aquaculture No 1, Dinh Bang 220000, Tu Son, Bac Ninh, Vietnam 4 Department of Analytical Chemistry, Faculty of Natural Sciences, Comenius University in Bratislava, Ilkovicova 6, SK-842 15 Bratislava, Slovakia * Correspondence: [email protected]; Tel.: +420-777318672; Fax: +420-387774634 Received: 19 May 2020; Accepted: 5 June 2020; Published: 10 June 2020 Abstract: Water from wastewater treatment plants contains concentrations of pharmaceutically active compounds as high as micrograms per liter, which can adversely affect fish health and behavior, and contaminate the food chain. Here, we tested the ability of the common carp hepatic S9 fraction to produce the main metabolites from citalopram, metoprolol, sertraline, and venlafaxine. Metabolism in fish S9 fractions was compared to that in sheep. The metabolism of citalopram was further studied in fish. -

Escitalopram Tablet, Film Coated Directrx ------ESCITALOPRAM
ESCITALOPRAM- escitalopram tablet, film coated DirectRX ---------- ESCITALOPRAM BOXED WARNING SECTION WARNINGS: SUICIDALITY AND ANTIDEPRESSANT DRUGS Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of escitalopram oxalate or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Escitalopram oxalate is not approved for use in pediatric patients less than 12 years of age. [See Warnings and Precautions: Clinical Worsening and Suicide Risk ( 5.1), Patient Counseling Information: Information for Patients ( 17.1), and Use in Specific Populations: Pediatric Use ( 8.4)]. INDICATIONS & USAGE SECTION 1.1 Major Depressive Disorder Escitalopram -

Pharmacogenetics of Antidepressant Response
Copyright 2007 by Eric James Peters ii ACKNOWLEDGEMENTS Knowledge is priceless. Perhaps this is because the process of acquiring it is painfully slow - entire careers and countless hours of work have been performed in hopes of adding just small pieces to our fragmented understanding of the natural world. Frustrations and setbacks abound, as experiments fail and assays stop working when needed most. But the prospect of improving human health, advancing a field, or simply being the first to know something has a certain appeal. What is clear is that knowledge cannot be pursued as a solo endeavor. I was fortunate to have the support of a tremendous group of colleagues, family and friends. Without them, I would not never made it through the process. First and foremost, I would like to thank Steve Hamilton. His guidance is the reason my graduate school career had the bright spots that it did. He has taught me that science, at its very core, is not about a single experiment or laboratory technique. Instead, it is about the pursuit of knowledge, and to be a successful scientist one cannot succumb to tunnel vision. I’ve spent many engaging hours in his office discussing such varied topics as genetics, psychiatry, and religion, and he has always encouraged any curiosity or interest that I felt a need to discuss, no matter how irrelevant it was to my thesis project. He has also taught me the art of presenting science that is both exciting and accessible to the audience, which is an invaluable tool for any independent investigator. -

Form Requisition
1327 Miller Road, Suite F Laboratory Testing Services Greenville, SC 29607 +1-864-568-8940 (o) Test Requisition: Toxicology +1-864-568-8942 (f) LuxorScientific.org Confidential An Innovative Scientific Solutions Company Requesting Provider & Insurance Information Patient Information Ordering Physician Client # Client Name Patient Name Client Street Address Client Phone Date of birth 侊 Male 侊 Female Patient’s Insurance Provider For This Test* Policy/Group No. Patient Address *Attach demo and 侊 Self Pay 侊 W/C Date of Injury Patient City/State/Zip Code front/back of card 侊 W/C Case Number Patient Phone Number ICD-10 Treatment Codes (include primary diagnosis code in “Other”) Explanation ܆ F10.20 ܆ F19.20 ܆ Z51.81 ܆ Z79.891 ܆ Identifies absence of prescribed medication ܆ F11.20 ܆ G89.4 ܆ Z91.19 ܆ Z79.899 ܆ Identifies undisclosed substances ܆ Other ܆ Identifies substances that contribute to adverse drug events or drug-drug interactions ܆ Baseline Test ܆ Periodic Monitoring ܆ Provides objectivity to the treatment plan ܆ High Risk Patient ܆ Confirmation Required ܆ Reinforces therapeutic compliance in the patient ܆ Targeted Testing ܆ Illicit Detection ܆ Other ܆ Inadequate Detection ܆ Best Practice Medications (attach patient’s medication list) ܆ Adderalů ܆ Celexa ܆ Dronabinol ܆ Hydromorphone ܆ Marinol ܆ Oxymorphone ܆ Seroquel ܆ Vicodin ܆ Adipex ܆ Cheratussin ܆ Duloxetine ܆ Kadian ܆ Meperidine ܆ Paroxetine ܆ Sertraline ܆ Vyvanse ܆ Alprazolam ܆ Citalopram ܆ Duragesic ܆ Keppra ܆ Methadone ܆ Paxil ܆ Soma ܆ Wellbutrin ܆ Ambien ܆ Clonazepam ܆ -
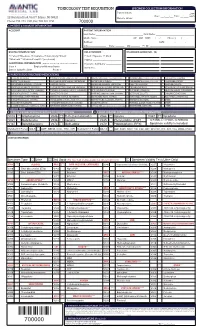
Toxicology Test Requisition Specimen Collection/Information
TOXICOLOGY TEST REQUISITION SPECIMEN COLLECTION/INFORMATION Patient Initials: ________________ ☐am 22 Meridian Road, Unit 7, Edison, NJ 08820 Date: ___/___/___ Time: __:____ ☐pm Labelc Donor’s Initials: ________________ Phone: 732-474-1120, Fax: 732-321-1150 700000 1. PATIENT & ACCOUNT INFORMATION ACCOUNT PATIENT INFORMATION Last Name: _____________________________________ First Name: ______________________________ Middle Name: __________________________ ☐F ☐M DOB: ___/___/___ Phone: (____)____ - ____ Address: __________________________________________________ SSN: _______ - ______ - ______ City:______________ State: _______ Zip:_______ Pt. ID: ___________________________________ BILLING INFORMATION RELATIONSHIP DIAGNOSIS CODES (ICD - 10) ☐ Patient ☐Medicare ☐ Insurance ☐ Auto Injury ☐Client ☐ Self ☐Spouse ☐ Child ☐Medicaid ☐ Workers Comp/PIP (see below) ☐Other _________________________ ADDITIONAL INFORMATION (Required for all Workers Comp or if no insurance card is attached) Insurance Company: ____________ Case # _____________ Employer/Attorney Name: _______________ Member # _____________________ Date of Injury/Accident: _______________ Phone # ______________ 2. PARENT DRUG (PRESCRIBED MEDICATIONS) ALPRAZOLAM (XANAX) CYCLOBENZAPRINE (AMRIX, FEXMID, FLEXTRIL) LORAZEPAM (ATIVAN, LORAZAPAM INTENSOL) OXYMORPHONE (OPANA IR, NUMORPHAN) VENLAFAXINE (EFFEXOR) AMITRIPTYLINE (ELAVIL) DESIPRAMINE (NORPRAMINE, PERTOFRANE) MAPROTILINE (LUDIOMIL) PHENOBARBITAL (LUMINAL, SOLFOTON) VILAZODONE (VIIBRYD) AMPHETAMINE (ADDERALL, VYVANSE) DIAZEPAM