Isolation of a Major Host Plant-Terpenoid and Its Probable
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United Bank of India - Latest CSP List
United Bank of India - Latest CSP List S.No. Village Name Base Branch District State BCA Name Contact No. Joining Date 1 GOPALPUR Bedaul Asli Muzaffarpur Bihar Abhay Kumar 9955962362 09.09.2011 2 BHARAT PATTI Bedaul Asli Muzaffarpur Bihar Nityanand Singh 9162065475 09.09.2011 3 THENGPUR Bahdinpur Muzaffarpur Bihar Vinay Kumar Sah 9939515448 09.09.2011 9199645686 4 TILAKPAKRI Basantpur patti Muzaffarpur Bihar Mukesh Kumar Thakur 9471819911 09.09.2011 8252459101 5 BANAULI Basantpur patti Muzaffarpur Bihar Chandrashekhar Sharma 9939876972 09.09.2011 6 BAHLOLPUR Bahdinpur Muzaffarpur Bihar Chandeshwar Ram 9572019378 09.09.2011 7 INDAULIA Bahdinpur Muzaffarpur Bihar Ravi Kumar 9955234908 09.09.2011 8 FIROZPUR Bahdinpur Muzaffarpur Bihar Dinesh Kumar 9934946160 09.09.2011 9 AYODHAPUR Basantpur patti Muzaffarpur Bihar Sabita Kumari 8809915794 09.09.2011 10 JAGDISHPUR Purshottampur Muzaffarpur Bihar Lalan kumar 9798660862 01.12.2011 11 BAHBAL BAZAR Minapur Muzaffarpur Bihar Abdhesh Kumar 8603374370 01.12.2011 12 DAUDPUR Gokula Muzaffarpur Bihar Md.Naushad alam 9939007864 01.12.2011 13 USTI Gokula Muzaffarpur Bihar Md.Amin Kaushar 8809175008 01.12.2011 14 BHAGWATPUR Gokula Muzaffarpur Bihar Satyandra kumar singh 9006499435 01.12.2011 15 BISUNPURKANTH Minapur Muzaffarpur Bihar Ram babu ray 9939177312 01.12.2011 16 DARHIPATTI Minapur Muzaffarpur Bihar Chandan kumar 9939264291 01.12.2011 17 KHEMAIPATTI Minapur Muzaffarpur Bihar Manish kumar 9631163935 01.12.2011 18 MANIKPUR Minapur Muzaffarpur Bihar Islam Ansari 9534508370 01.12.2011 19 PHULWARIA -
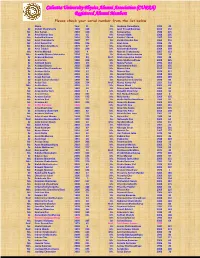
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please Check Your Serial Number from the List Below Name Year Sl
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please check your serial number from the list below Name Year Sl. Dr. Joydeep Chowdhury 1993 45 Dr. Abhijit Chakraborty 1990 128 Mr. Jyoti Prasad Banerjee 2010 152 Mr. Abir Sarkar 2010 150 Dr. Kalpana Das 1988 215 Dr. Amal Kumar Das 1991 15 Mr. Kartick Malik 2008 205 Ms. Ambalika Biswas 2010 176 Prof. Kartik C Ghosh 1987 109 Mr. Amit Chakraborty 2007 77 Dr. Kartik Chandra Das 1960 210 Mr. Amit Kumar Pal 2006 136 Dr. Keya Bose 1986 25 Mr. Amit Roy Chowdhury 1979 47 Ms. Keya Chanda 2006 148 Dr. Amit Tribedi 2002 228 Mr. Krishnendu Nandy 2009 209 Ms. Amrita Mandal 2005 4 Mr. Mainak Chakraborty 2007 153 Mrs. Anamika Manna Majumder 2004 95 Dr. Maitree Bhattacharyya 1983 16 Dr. Anasuya Barman 2000 84 Prof. Maitreyee Saha Sarkar 1982 48 Dr. Anima Sen 1968 212 Ms. Mala Mukhopadhyay 2008 225 Dr. Animesh Kuley 2003 29 Dr. Malay Purkait 1992 144 Dr. Anindya Biswas 2002 188 Mr. Manabendra Kuiri 2010 155 Ms. Anindya Roy Chowdhury 2003 63 Mr. Manas Saha 2010 160 Dr. Anirban Guha 2000 57 Dr. Manasi Das 1974 117 Dr. Anirban Saha 2003 51 Dr. Manik Pradhan 1998 129 Dr. Anjan Barman 1990 66 Ms. Manjari Gupta 2006 189 Dr. Anjan Kumar Chandra 1999 98 Dr. Manjusha Sinha (Bera) 1970 89 Dr. Ankan Das 2000 224 Prof. Manoj Kumar Pal 1951 218 Mrs. Ankita Bose 2003 52 Mr. Manoj Marik 2005 81 Dr. Ansuman Lahiri 1982 39 Dr. Manorama Chatterjee 1982 44 Mr. Anup Kumar Bera 2004 3 Mr. -
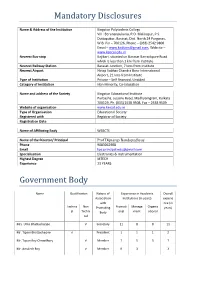
Mandatory Disclosures
Mandatory Disclosures Name & Address of the Institution Kingston Polytechnic College Vill : Berunanpukuria, P.O. Malikapur, P.S. Duttapukur, Barasat, Dist. North 24 Parganas, W.B. Pin – 700126, Phone – (033) 2542 9800 Email – [email protected], Website – www.kpccal.edu.in Nearest Bus-stop Kajibari, situated on Barasat Barrackpore Road which is less than 1 km from Institute Nearest Railway Station Barasat Junction, 7 kms from Institute Nearest Airport Netaji Subhas Chandra Bose International Airport, 21 kms from Institute Type of Institution Private – Self financed, Unaided Category of Institution Non Minority, Co-Education Name and address of the Society Kingston Educational Institute Purbasha, Jessore Road, Madhyamgram, Kolkata 700129, Ph. (033) 2538 9508, Fax – 2538 9509 Website of organisation www.keical.edu.in Type of Organisation Educational Society Registered with Registrar of Society Registration Date Name of Affiliating Body WBSCTE Name of the Director/ Principal Prof Diptarup Bandopadhyay Phone 9083062908 Email [email protected] Specialisation Electronics & Instrumentation Highest Degree MTECH Experience 23 YEARS Government Body Name Qualification Nature of Experience in Academic Overall Association Institutions (in years) experie with nce (in Technic Non Promoting Promoti Manage Organiz years) al Techni Body onal ment ational cal Mrs. Uma Bhattacharjee √ Secretary 11 8 8 11 Mr. Tipam Bhattacharjee √ President 1 1 1 2 Mr. Tapan Roy Chowdhury √ Member 7 5 5 7 Mr. Amalesh Roy √ Member 0 3 - 3 Dr. Manishankar Chakraborty √ Member 3 0 1 4 WBSCTVE&SD Nominating N.A. N.A. - N.A. √ Member AICTE(ERO) Nominating N.A. N.A. - N.A. √ Member Prof. J.P. Bandopadhyay √ Member 0 0 8 8 √ Member 0 0 13 13 Mr. -

WEST BENGAL STATE UNIVERSITY Berunanpukuria, Malikapur Barasat 24 Parganas (North), Kolkata - 700 126 Phone: (033) 2524 1975/1976/1978/1979 Fax: (033) 2524 1977
WEST BENGAL STATE UNIVERSITY Berunanpukuria, Malikapur Barasat 24 Parganas (North), Kolkata - 700 126 Phone: (033) 2524 1975/1976/1978/1979 Fax: (033) 2524 1977 P' -- WBSUlReg/ Affiliation/Cert./1931115-16 am:«. ....·07,o·1-:2016··········· IRs! 'No: . TO WHOM IT MAY CONCERN This is to certify that Gandhi Centenary B.T College, Habra, North 24 Parganas, West Bengal was affiliated to the Calcutta UniversitY since 1968 and subsequently affiliated to the West Bengal State University from 2008 vide Govt. Notification No. 300- Edn (U)/EHlIl U-38/08, dated 26th MaY, 2008 and the college recognized bY the University Grants Commission (under 2(t) and 12 B) and the following Courses/Subjects are taught in the said college as per approval. The Course is valid as per NCTE norms. Sl. Name off the Course(s) and Duration Affiliation Period of ValiditY No. Permanent I Temporary for the Year( s) (i) B.Ed .... Permanent (i) M.Ed Proposal submitted to .... NCTE Registrar (Officiating) West Bengal State UniversitY Dr. Ramanuj GangulY Registrar (OffiCiating) West Bengal State University Barasat, Kolkata-700126 WEST BENGAL STATE UNIVERSITY Berunanpukuria, Malikapur .Barasat 24 Parganas (North), Kolkata - 700 126 Phone: (033) 2524 1975/ 1976/ 1978/ 1979 Fax: (033) 2524 1977 eg/AffiliationiCert.l19311l5-16 Date : ....-07.O-L2B 16··········· CRsj:No: . TO WHOM IT MAY CONCERN This is to certify that Gandhi Centenary B.T College, Habra, North 24 Parganas, West Bengal was affiliated to the Calcutta University since 1968 and subsequently affiliated to the West Bengal State University from 2008 vide Govt. Notification No. 300- Edn (U)/EHlIl U-38/08, dated 26th May, 2008 and the college recognized by the University Grants Commission (under 2(t) and 12 B) and the foilowing Courses/Subjects are taught in the said college as per approval. -
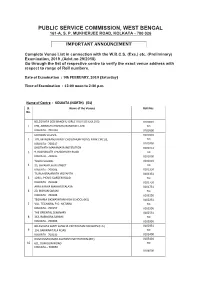
Complete Names and Addresses of the Venues of the Upcoming West
PUBLIC SERVICE COMMISSION, WEST BENGAL 161-A, S. P. MUKHERJEE ROAD, KOLKATA - 700 026 IMPORTANT ANNOUNCEMENT Complete Venue List in connection with the W.B.C.S. (Exe.) etc. (Preliminary) Examination, 2019 ,(Advt.no 29/2018) Go through the list of respective centre to verify the exact venue address with respect to range of Roll numbers. Date of Examination : 9th FEBRUARY, 2019 (Saturday) Time of Examination : 12:00 noon to 2:30 p.m. Name of Centre : KOLKATA (NORTH) (01) Sl. Name of the Venues Roll Nos. No. BELEGHATA DESHBANDHU GIRLS' HIGH SCHOOL (HS) 0100001 1 69B, ABINASH CHANDRA BANERJEE LANE TO KOLKATA - 700 010 0100500 MODERN SCHOOL 0100501 2 17B, MANORANJAN ROY CHOUDHURY ROAD, PARK CIRCUS, TO KOLKATA - 700017 0100750 BHUTNATH MAHAMAYA INSTITUTION 0100751 3 9, RADHANATH CHOWDHURY ROAD TO KOLKATA - 700015 0101000 TOWN SCHOOL 0101001 4 33, SHYAMPUKUR STREET TO KOLKATA - 700004 0101350 TILJALA BRAJANATH VIDYAPITH 0101351 5 129/1, PICNIC GARDEN ROAD TO KOLKATA - 700039 0101750 ARYA KANYA MAHAVIDYALAYA 0101751 6 20, BIDHAN SARANI TO KOLKATA - 700006 0102250 TEGHARIA SIKSHAYATAN HIGH SCHOOL (HS) 0102251 7 VILL. TEGHARIA, P.O. HATIARA TO KOLKATA - 700157 0102550 THE ORIENTAL SEMINARY 0102551 8 363, RABINDRA SARANI TO KOLKATA - 700006 0102950 BELEGHATA SANTI SANGHA VIDYAYATAN FOR BOYS (H.S.) 0102951 9 1/4, BARWARITALA ROAD TO KOLKATA - 700010 0103400 DUM DUM KUMAR ASUTOSH INSTITUTIOIN (BR.) 0103401 10 6/1, DUM DUM ROAD TO KOLKATA – 700030 0104000 KHANNA HIGH SCHOOL (H.S.) SUB-CENTRE 'A' 0104001 11 9, SHIVKUMAR KHANNA SARANI TO KOLKATA – 700015 0104350 KHANNA HIGH SCHOOL (H.S.) SUB-CENTRE 'B'; 0104351 12 9, SHIVKUMAR KHANNA SARANI TO KOLKATA - 700015 0104700 BELEGHATA SANTI SANGHA VIDYAYATAN FOR GIRLS 0104701 13 1/8, BAROWARITALA ROAD TO KOLKATA - 700010 0105250 BELEGHATA DESHBANDHU HIGH SCHOOL (BR.) 0105251 14 17/2E, BELEGHATA MAIN ROAD TO KOLKATA – 700010 0105700 BAGMARI MANIKTALA GOVT. -

Barasat Today
A INITIA Fortune Realty is a Kolkata-based real estate companyTIVEthe beauty of life and enhance the quality of living’. founded in 1985 by the veteran Dr. R. S. Bhartia. With This reflects in the Group’s evolving, premium and BARASA his world 35 years of experience, immense knowledge in various class real estate projects across Kolkata. The Group fields and immaculate expertise, Dr. Bhartia has carved believes that in today’s fast-paced life, the beauty of life out a niche for Fortune Realty in the Kolkata real estate is manifested in moments and precious time spent in sector. Mr. Padmanabh Bhartia has continued to take quiet, tranquility and peace in the company of loved T the ones. Fortune Group a step ahead and set benchmarks A real estate company in Kolkata, Fortune amongThe main motto of the Fortune Realty is ‘to Realty, Kolkataaccentuate housings and properties. deals with residential and commercial projects. TODA NH 35 Madhyamgram Phone: 9748570000 Phone: 9231433798 Y BARASAT TRENDING AND RETAIL. Barasat - NH34 Phone: 8584919999 THE STORY OF A FAVOURITE TOWNBARASAT ADDRESS OF NEW HOME BARASAT'S .CALLED Chetla Central Road, LEGENDARY BUYERS. Kolkata – 700027 KALI PUJOS. Phone: 9831718192 Head Office: Gillander House, B-Block 1st Floor, 8 Netaji Subhas Road, Kolkata 700001 Contact : 92314 33798, +91 33 4019 9595 | [email protected] | www.fortunerealty.in ARASAT is a city in Greater Kolkata, West Bengal India and is the district headquarters of North 24 Parganas. It is a part Bof Authority. Barasat Municipality, Barast District Court and Zilla the area covered by Kolkata Metropolitan Development Parishad (District Council) are the major administrative establishments of Barasat. -

West Bengal State University Berunanpukuria, Barasat, Kolkata
West Bengal State University Berunanpukuria, Barasat, Kolkata 700126 Curricula for Three-Year Under-Graduate Advanced (Honours) [EDCA] and General Degree [EDCG] Programmes in EDUCATION 1. Preamble The present Curricula for three-year Advanced (Honours) and General Degree Programmes in Education have been designed following the recommendations of national documents, viz., NPE (1986), POA (1992), NCF (2005) and CRR of UGC. Simultaneously, Curricula of other State Universities and the unique socio-cultural nature of the University jurisdiction have also been considered in course of developing the present curricular framework. Since the establishment of the University in 2008 this is the first attempt in developing curricula for three-year Advanced (Honours) and General Degree Programmes in Education to be effective from the session 2013-14. The main rationale behind the present curricular frame is to develop the Educational base as a liberal academic discipline among the under-graduate learners both in Advanced (Honours) and General levels. 2. UG Degree Programmes in Education The University offers two types of UG programmes in the broad domain of liberal Education discipline through its affiliated Degree Colleges – (i) Three-year Advanced (Honours) Degree Programme and (ii) Three-year General Degree Programme. 1 2.1 The Advanced (Honours) Degree Programme in Education [EDCA] 2.1.1 The Course Structure: Course Suggested Marks Class-hour Year No. Course Title Group per Week Allotted EDCA Philosophical and A. Educational 03 50 Sociological 01 Foundations of Philosophy Education B. Educational 03 50 1st Sociology Educational A. Educational 03 50 Psychology and 02 Psychology Pedagogy B. Pedagogy 03 50 Development of A. -

TRAINING REPORT Malikapur Barasat, Berunanpukuria Kolkata, West Bengal
TRAINING REPORT Malikapur Barasat, Berunanpukuria Kolkata, West Bengal Duration : 2 day training program on Capacity Building in Fruit Ripening Date : 19th & 20th August 2015 at Berunanpukuria, Kolkata, West Bengal S. No Particulars Remarks 1 Detailed curriculum Enclosed 2 List of faculty with brief profile Enclosed 3 Program Schedule 4 List of trainees Enclosed 5 List of representatives of Ripening Chambers participated Enclosed a) Ripening chambers in M/S Keventer Fresh Ltd. Enclosed b) Opinion of the representatives of ripening chambers where training is Enclosed required. 6 Feedback from university Enclosed 7 Feedback from trainees Enclosed 8 Feedback from faculty Enclosed 9 Press note Enclosed 10 Media attention received: Print media Enclosed 11 Photos taken during training program Enclosed a) First day of training program inauguration and classroom photos Enclosed b) Hands on training & Field visit to ripening chamber Enclosed Malikapur Barasat, Berunanpukuria, West Bengal - DETAILED CURRICULUM INAUGURATION - GUESTS ON DIAS 1 Mr. Asim Kumar Bhadra Director, Kingston Polytechnic College 2 Mrs. Uma Bhattacharya Secretary, Kingston Polytechnic College 3 Mr Ranjan Roy Manager, SBI, Kolkata 4 Mr. Dipankar Sur Project Manager, ICE – Centre of Excellence. 5 Dr. Jay Prakash Chairman, Kingston Polytechnic College Bandhopadhyay 6. Swagata Chakraborty Training Officer, Kingston Polytechnic College LIST OF FACULTY WITH BRIEF PROFILE Dr. Jay Prakash Bandhopadhyay PhD (Tech), FIETE, SMIEEE (USA), the Chairman (Academic) of Kingston Educational Institute, UGC Emeritus Professor in the Institute of Radio Physics & Electronics, University of Calcutta and Director of CMSDS Mr. Asim Kumar Bhadra, Director, Kingston Polytechnic College B.Tech and M.Tech from Sivpur, Hawrah Ex Chairman of West Bengal state council of technical education. -

Proceedings of 229 Meeting of the Erc-Ncte Held on 11
Proceedings of 229th Meeting of ERC-NCTE held on 11th -12th January, 2017 PROCEEDINGS OF 229th MEETING OF THE ERC-NCTE HELD ON 11th - 12th January, 2017 The 229th Meeting of the Eastern Regional Committee (ERC), National Council for Teacher Education (NCTE) was convened on 11th - 12th January, 2017 at Conference Hall, ERC, NCTE, 15, Neelakantha Nagar, Nayapalli, Bhubaneswar. The Chairperson, Members and Convener as listed below were present at the time of the 229th ERC Meeting. Prof. Brajanath Kundu - Chairperson Dr. Deva Kumar Dutta - State Representative of Assam Prof. Vanlalhruaii - State Representative of Mizoram Ms. Mansi Nimbhal - State Representative of Odisha Dr. Tilottama Senapati - State Representative of Odisha Shri Pradeep Kumar Yadav - Regional Director (I/C), ERC & Convener At the outset, the Regional Director welcomed all the members of Committee and apprised them about the provisions of the NCTE Act, Rules, Regulations and Guidelines issued from time to time. With the permission of Chairperson, Shri Pradeep Kumar Yadav (Convener) presented the Agenda before the Committee for its due consideration and appropriate decision. Sl.No. Item No. Content Confirmation of the Proceedings of 228th Meeting held on 29th – 30th December, 2016 ER-229.1 at Bhubaneswar. Confirmed with following rectification in respect of item No. 228.8.3 (Sl. No. 96 in the proceeding: The decision of the Appeal Committee noted by the ERC and decided that the name of the institution viz. Kalimuddin Smriti B.Ed. College, Plot No- 289, 431, Street No.- NH- 34, Vill- Halalpur, PS- Runia, Tehsil/Taluka- Tajpur, Town/City- Raiganj, Dist- Uttar Dinajpur, West Bengal- 733156 (B.Ed.) (ERCAPP2510) be changed as “Uttar Dinajpur Primary Teachers Training Institute, Plot No- 289, 431, Street No.- NH-34, Vill- Halalpur, PS- Runia, Tehsil/Taluka- Tajpur, Town/City- Raiganj, Dist- Uttar Dinajpur, West Bengal- 733156”. -
Socio Economic Change of Barasat: a Case Study Berunanpukuria
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 20, Issue 10, Ver. VI (Oct. 2015) PP 80-87 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org Socio Economic Change of Barasat: A Case Study Berunanpukuria 1.Mr.Mithu Roy 2.Mr.Chandan Das Dept. of Geography, Naba Barrackpore Prafulla Chandra Mahavidalay Dept. of Geography, Sundarban Mahavidyalaya(Kakdwip,South 24 Pgs) Abstract: Berunanpukuria is the largest mouza in the Icchapur Nilganj Gram Panchayet of Barasat-I Block in North 24 parganas in West Bengal. Berunanpukuria is situated on the middle of Barrackpur-Barasat highway in the vicinity of Barasat town. The village is large with an area of 152.92 sq.km. It has a population of 2162(according to 2001 census.) In the past(before 10 years) time the village was fully dependent on agriculture and fishery. But after the education institutions were set up the village become a developed village and in future it will become educational hub as well as an urban area. Here the study mainly focuses on the identification of socio-economic and cultural transformation of the village due to set up of education institution (W.B.S.U & Kingston Educational institute). When the Kingston Educational Institute (Technical engineering college) was set up in 2004 the transformation started and then after 2009 when the West Bengal State University was established a huge change is seen in transportation facilities, income level, change in land use, social amenities of study area, as well as density of population per sq-km, increase of total population, house construction, employment both male and female, health care facilities, increase of literacy, change of food habit, dress patterns, house amenities, nutrition distribution, bank account, etc.Therefore an attempt has been made here to identify the socio-economic and cultural transformation of the village and its benefit. -
Applications Received by UCO Bank Sl No Name of Student Date of Birth Gender Category Mobile No
Applications received by UCO Bank Sl No Name of Student Date Of Birth Gender Category Mobile No. Annual Name of Instituion Address of Instituion Whether Marks Loan amount Family NAAC/Q Obtained (in required Income AC % in UG/PG available Level last studied) 1 ABHIJIT 12-12-1995 MALE UR 9612278549 72,000.00 KAZI NAZRUL ISLAM VILL+PO: PANCHGRAM, 5.2 2,00,000.00 CHOWDHURY TEACHER TRAINING PS: NABAGRAM, DIST: COLLEGE` MURSHIDABAD 2 AKASH DAS 11-11-1996 MALE SC 7005235405 60,000.00 MAHULA SRI KANACHI, BIRBHUM, SGPA : 5.87 1,50,00.00 RAMKRISHNAN DIST: RAMPURHAT, WEST TEACHERS TRAINING BENGAL, 731224 INSTITUTE 3 AMRIT SAHA 02-01-1997 MALE UR 8837235067 30,000.00 RAMKRISHNA CHANDIPUR, PO: PINPUR, CGPA: 5.27 2,00,000.00 VEDANTA MISSION PS: ULUBERIA, DIST: TEACHERS ACADEMY HOWRAH, WEST BENGAL 4 ANAMIKA 20-04-1993 FEMALE OBC 8414936550 66,000.00 OLIVE ACADEMY VILL: KANCHANPUR 57% 1,50,000.00 MAJUMDER (BAGPARA), PO: NATUNGANG, PS: RAGHUNATHGANJ, MURSHIDABAD, WEST 5 GANESH SAHA 21-11-1995 MALE UR 8794616303 60,000.00 SWAMI VILL+PO+PS:BENGAL, 742227 YES 55% 2,00,000.00 VIVEKANANDA B. ED CHANDRAPUR, DIST: COLLEGE BIRBHUM, WEST BENGAL, 731126 6 HANIF MIAH 01-05-1995 MALE UR 7627900700 60,000.00 KABI NAZRUL MAHA 5.19% 2,00,000.00 VIDYALAYA 7 KISHAN DAS 03-01-1997 MALE SC 9774683748 72,000 EHIAPUR B. ED VILL+PO: EHIAPUR, PS: 54.50% 2,00,000.00 COLLEGE KETUGRAM, DIST: BURDWAN, WEST BENGAL 8 MITHUN 07-02-1997 MALE OBC 7005771150 96,000.00 MAHULA SRI KANACHI, BIRBHUM, UG-51% 1,50,000.00 DEBNATH RAMKRISHNAN DIST: RAMPURHAT, WEST TEACHERS TRAINING BENGAL, 731224 INSTITUTE 9 PAYEL 17-07-1976 FEMALE SC 8787471143 60,000.00 BAGNAN TEACHER'S PO: MUGKALYAN, PS: NAAC BA(H) - 56% 1,60,000.00 CHOWDHURY TRAINING COLLEGE BAGNAN, DIST: HOWRAH WEST BENGAL, 711312 10 PIJUSH DATTA 20-06-1992 MALE OBC 7085865651 96,000.00 JAGAT BANHU VILL: MAHINPUR, PO: 52.37% 1,50,000.00 TEACHER TRAINING AZIMGANJ, COLLEGE MURSHIDABAD, WEST BENGAL, 742122 11 PRANOY 23-04-1997 MALE UR 8837300452 70,000.00 M.M. -

Report WEBINAR on CARE to BIODIVERSITY on the Occasion of World Environment Day-5Th June, 2020 Venue: Youtube & Facebook
Report WEBINAR on CARE TO BIODIVERSITY On the occasion of World Environment Day-5th June, 2020 Venue: Youtube & Facebook Organised by: ENVIS RP on Environmental Biotechnology, University of Kalyani Under the aegis of University of Kalyani ("A" Grade Accredited by NAAC) West Bengal, India Sponsored by: MoEF & CC, Govt. of India Webinar Brochure was widely circulated through DESKU- ENVIS website, facebook page, Whatsapp groups and E.mails 2 Content Sl. Particulars Page No No 1 Aim of the programme 4 2 Welcome Address by Prof. (Dr.) Ashis K Panigrahi, 7 Coordinator, ENVIS RP, KU. 3 Inaugural Address: Prof. (Dr.) Sankar Kumar Ghosh, 10 Hon’ble Vice Chancellor, KU. 4 List of Resource Persons 13 5 Participants details 14 6 Feedback of Participants 17 7 Invited speakers 22 8 Vote of Thanks 32 9 Photo Gallery 34 10 Annexure (List of Participants) 40 3 Invitation Aim of the Webinar Webinars are events held 100% online. This enables you to reach a large and specific audience. Members of the target group participate live via a PC, Mac, tablet or smartphone, wherever they are. And if they are unable to join the webinar live, they can watch the recorded broadcast afterwards instead. This webinar is organised in the framework of the coronavirus disease (COVID-19) outbreak. Seven months into the discovery of the COVID- 19 virus in Wuhan, almost half of humanity is under lockdown. Thousands of events were cancelled worldwide due to the Corona virus and in a COVID-ridden world; gatherings of any kind can be very dangerous for all parties.