ACIP Recommended Immunization Schedule for Adults Aged 19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite. -

The Study of Thimerosal and Autism
The Study of Thimerosal and Autism Documentation and Codebook for the Child Vaccination Histories File: Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD September 3, 2010 Chicago, IL Prepared for Frank DeStefano National Immunization Program Centers for Disease Control and Prevention Atlanta, GA 30329 Prepared by Cristofer Price Yeqin He Abt Associates Inc. Suite 800 North 4550 Montgomery Avenue Bethesda, MD 20814-3343 This documentation was prepared by Cristofer Price and Yeqin He of Abt Associates Inc. for the Immunization Safety Office (ISO) of the Centers for Disease Control and Prevention (CDC) Atlanta, GA 30333. Questions about the documentation, substantive questions, or technical issues regarding the data file should be directed to the CDC ISO, MS D26, 1600 Clifton Road, Atlanta, Georgia 30333 (404-639-8256). Suggested Citation for Study of Thimerosal and Autism Public Use Data Set: Price, C.S., He, Y. (2010) The Study of Thimerosal and Autism: Data Set Documentation. Bethesda MD: Abt Associates, Inc; Prepared for the National Immunization Program Centers for Disease Control and Prevention Atlanta, GA Abt Associates Inc. 1 Documentation and Codebook for the Child Vaccination Histories File 1. Introduction to the Child Vaccination Histories File............................................... 2 2. File Formats and Variable Descriptions................................................................... 4 1. Introduction to the Child Vaccination Histories File The Child Vaccination Histories File contains the data that were used to construct the measures of early childhood (postnatal) exposure to ethylmercury from thimerosal- containing vaccines and immune globulin preparations received by the study children during the age range spanning birth to 20 months. The Child Vaccination Histories File is provided with the public use data in order to make the calculation of exposure amounts transparent, and so that analysts have the potential to calculate alternative measures of exposure1. -

Gsk Vaccines in 2010
GSK VACCINES IN 2010 Thomas Breuer, MD, MSc Senior Vice President Head of Global Vaccines Development GSK Biologicals Vaccines business characteristics Few global players and high barriers to entry – Complex manufacturing – Large scale investment Long product life cycles – Complex intellectual property High probability of R&D success – 70% post-POC New technology/novel products Better pricing for newer vaccines – HPV vaccines (Cervarix, Gardasil) – Pneumococcal vaccines (Synflorix, Prevnar-13) Operating margin comparable to pharmaceutical products Heightened awareness New markets 2 Research & development timelines Identify Produce Pre-Clinical Proof of Registration/ Phase I Phase II Phase III File Antigens Antigens Testing Concept Post Marketing Research (inc. Immunology) Pre-Clinical Development (inc. Formulation Science) Clinical Development (inc. Post Marketing Surveillance) Transfer Process to Manufacturing Build Facility x x Up to $10-20M Up to $50-100M $500M - $1B x x x 1-10 yrs 2-3 yrs 2-4 yrs > 1 yr GSK vaccines business 2009 sales £3.7 billion (+30%) +19% CAGR excl. H1N1 Vaccines represent 13% since 2005 of total GSK sales Sale s (£m) 4000 3500 Recent approvals: 3000 US: Cervarix 2500 EU: Synflorix 2000 Pandemic: Pandemrix; Arepanrix 1500 1000 500 0 2005 2006 2007 2008 2009 Increased Emerging Market presence Growth rate is CER 5 GSK vaccines: fastest growing part of GSK in 2009 2009 Sales Share Growth (CER) Respiratory £ 6,977m 25% +5% Consumer £ 4,654m 16% +7% Anti-virals £ 4,150m 15% +12% Vaccines £ 3,706m 13% +30% CV & Urogenital -
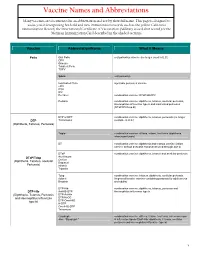
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand .................................................................... -

Twinrix (Hepatitis a & B Combination)
TWINRIX (HEPATITIS A & B COMBINATION) Disease Risk & Transmission Hepatitis A (HAV) is a highly contagious liver disease with the common mode of transmission being person to person. Hepatitis A is found in the stool of infected persons and infection may be spread by contaminated water or food, by infected food handlers, after breakdown in sanitary conditions, or after floods or natural disasters, by ingestion of raw or uncooked shellfish from contaminated water, or by needle transmission (sharing needles, blood transfusions from infected persons), in daycare centers, during anal intercourse and during travel to countries in the world with poor hygienic conditions. A person who has Hepatitis A can easily spread on to others within the same household or dormitory. Symptoms include mild, flu-like symptoms, jaundice, decreased appetite, nausea and stomach pains, and diarrhea Hepatitis B (HBV) is an infection of the liver caused by the Hepatitis B virus. Hepatitis B is spread through contact with the blood of an infected person. Between 6-10 of every 100 young adults who have Hepatitis B become chronic carriers (have Hepatitis B in their blood for 6 or more months) and may be able to spread the infection to others for a long time. Hepatitis B is highly infective if there is contact with infected blood or serum through puncture wounds, splashes into the mucous membranes, contamination of open wounds, and injecting illegal drugs. It can be transmitted sexually, during birth and by sharing personal items such as razors and toothbrushes with an infected person. Symptoms include loss of appetite, tiredness, diarrhea & vomiting, pain in muscles and joints, dark urine & skin rashes. -

Hepatitis a Frequently Asked Questions
HEALTH NOTIFICATION FOR SOUTHERN NEVADA HEPATITIS A FREQUENTLY ASKED QUESTIONS WHAT ARE THE RECOMMENDATIONS is considered to be around 95 percent effective, that FOR HEPATITIS A VACCINE? protection will eventually begin to decrease and a Centers for Disease Control and Prevention (CDC) second shot boosts immunity for between 20 and 40 routinely recommends hepatitis A vaccination years, according to the CDC. for all children, for people who are at increased You can get a combination vaccine to protect you risk for infection, and for any people who want to against both hepatitis A and B. Check with your obtain immunity. health care provider. WHO SHOULD GET VACCINATED? CAN I GET MY HEPATITIS A SHOT During this current hepatitis A outbreak, the Health WHEN I GET OTHER IMMUNIZATIONS? District is working with community partners to You can receive the hepatitis A vaccine when you vaccinate people who are more likely to be infected receive other immunizations. The injection site will including people who use injection or non-injection be different. drugs (legal or illicit), people who are experiencing homelessness, and people who have recently been in IS THE HEPATITIS A VACCINE EFFECTIVE? jail or prison. Yes, the hepatitis A vaccine is highly effective in The CDC recommends hepatitis A vaccination for preventing hepatitis A virus infection. A second the following people: hepatitis A shot results in long-term protection. ¡ • All children at age of 1 year ¡ • People who are traveling to or working in countries IS THE HEPATITIS A VACCINE SAFE? where hepatitis A is common Yes, the hepatitis A vaccine is safe. -

Vaccine-Associated Paralytic Poliomyelitis and BCG-Osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013
Morbidity and Mortality Weekly Report Weekly / Vol. 63 / No. 33 August 22, 2014 Vaccine-Associated Paralytic Poliomyelitis and BCG-osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013 Robert Trimble, MD1, Jane Atkins, MD2, Troy C. Quigg, DO3, Cara C. Burns, PhD4, Gregory S. Wallace, MD4, Mary Thomas, MBBS5, Anil T. Mangla, PhD5, Anthony J. Infante, MD, PhD1 (Author affiliations at end of text) Poliovirus transmission has been eliminated in most of developed VAPP (2). A case of immunodeficiency-associated the world through the use of inactivated poliovirus vaccine vaccine-derived poliovirus (iVDPV) infection, without paraly- (IPV) and live, attenuated oral poliovirus vaccine (OPV). In sis, was diagnosed in an unvaccinated child with SCIDS in the United States, use of OPV was discontinued by the year 2005, but the source of the virus could not be definitively 2000 because of the potential for vaccine-associated paralytic identified (3). A woman in Minnesota aged 44 years with polio (VAPP); an average of eight cases were reported each long-standing common variable immunodeficiency died after year in the United States during 1980–2000 (1). Polio eradi- developing VAPP in 2009 (4). She was probably infected when cation efforts in other parts of the world continue to rely on her child received OPV approximately 12 years earlier. Case OPV to take advantage of transmission of poliovirus vaccine reports and cohort studies from several countries other than strains to unvaccinated persons in the population, lower cost, the United States demonstrate the continued occurrence of and ease of administration. In 2013, an infant aged 7 months iVDPVs and the need for ongoing surveillance (5). -

Global Advisory Committee on Vaccine Safety (GACVS)
Global Advisory Committee on Vaccine Safety (GACVS) Report on GACVS meeting June 2013 1 | GACVS June 2013 report Topics Discussed ! Pentavalent vaccine in 4 Asian Countries ! Zoster vaccine safety and varicella vaccine safety in immunocompromised populations ! Immunization during pregnancy ! Yellow fever vaccine safety during mass immunization campaigns in sub-Saharan Africa ! Safety profile: Japanese encephalitis vaccines ! Update: human papillomavirus vaccines ! Update: pandemic influenza vaccine (Pandemrix®) and narcolepsy 2 | GACVS June 2013 report Progressive pentavalent vaccine introduction in 4 Asian Countries ! Sri Lanka (Crucell, Jan 2008): – Within 3 months, 4 deaths and 24 suspected HHE: precautionary suspension of initial lot. – 1 death following immunization April 2009: vaccine suspended, DTwP and Hep B resumed. ! Bhutan (Panacea, Sep 2009) – 5 cases of encephalopathy and/or meningoencephalitis lead to suspended vaccination 23 Oct 2009. (Subsequently, 4 serious AEFI were identified and investigated). ! India (Serum Institute of India, Tamil Nadu and Kerala, Dec 2011; Goa, Pondicherry, Karnataka, Haryana, Jammu and Kashmir, Gujarat and Delhi from Q3 2012 – Q1 2013) – To date, 83 AEFI cases reported, some associated with mortality. ! Vietnam (Crucell, Jun 2010 - May 2013) – 43 serious AEFI investigated, including 27 with fatal outcome. – Following 9 deaths following vaccination reported Dec 2012 - March 2013: vaccine suspended. 3 | GACVS June 2013 report GACVS analysis of common features among countries experiencing significant vaccine safety concerns ! Vaccination programmes are well established and achieves high coverage ! Vaccine introduction was accompanied by thorough training of health-care staff on benefits and risks of vaccine ! Sri Lanka and Bhutan: discontinuation and resumption of pentavalent vaccine did not significantly modify pattern of serious AEFI with previously utilized vaccines ! Limitations in all 4 countries: – Incomplete clinical information complicated causality assessment.