Patients with Sensory Symptoms of Acral Paresthesia: Routine Laboratory Findings in Neuromuscular Consultation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Livedoid Vasculopathy Associated with Peripheral Neuropathy: a Report of Two Cases* Vasculopatia Livedoide Associada a Neuropatia Periférica: Relato De Dois Casos
CASE REPORT 227 s Livedoid vasculopathy associated with peripheral neuropathy: a report of two cases* Vasculopatia livedoide associada a neuropatia periférica: relato de dois casos Mariana Quirino Tubone1 Gabriela Fortes Escobar1 Juliano Peruzzo1 Pedro Schestatsky2 Gabriela Maldonado3 DOI: http://dx.doi.org/10.1590/abd1806-4841.20132363 Abstract: Livedoid vasculopathy (LV) is a chronic and recurrent disease consisting of livedo reticularis and sym- metric ulcerations, primarily located on the lower extremities, which heal slowly and leave an atrophic white scar ("atrophie blanche"). Neurological involvment is rare and presumed to be secondary to the ischemia from vascu- lar thrombosis of the vasa nervorum. Laboratory evaluation is needed to exclude secondary causes such as hyper- coagulable states, autoimmune disorders and neoplasms. We present two patients with a rare association of peripheral neuropathy and LV, thereby highlighting the importance of a multidisciplinary approach to reach the correct diagnosis. Keywords: Livedo reticularis; Mononeuropathies; Polyneuropathies; Skin diseases, vascular Resumo: Vasculopatia livedoide é uma doença crônica e recorrente caracterizada por livedo reticular e úlceras simétricas nos membros inferiores, que cicatrizam e deixam uma cicatriz branca atrófica ("atrophie blanche"). Envolvimento neurológico é raro e está provavelmente associado a isquemia pela trombose dos vasa nervorum. Avaliação laboratorial é indicada com o intuito de excluir causas secundárias como estados de hipercoagulabili- dade, doenças autoimunes e neoplasias. Apresentamos dois pacientes com uma rara associação de vasculopatia livedoide com neuropatia periférica, enfatizando a importância de uma abordagem multidisciplinar na busca do diagnóstico correto. Palavras-chave: Dermatopatias vasculares; Livedo reticular; Mononeuropatias; Polineuropatias INTRODUCTION Livedoid vasculopathy (LV) is a chronic and resentation of the dermo-hypodermic junction, was recurrent disease, usually restricted to the skin, and compatible with LV. -

Cerebral Venous Thrombosis and Livedo Reticularis in a Case with MTHFR 677TT Homozygote
Journal of Clinical Neurology / Volume 2 / June, 2006 Case Report Cerebral Venous Thrombosis and Livedo Reticularis in a Case with MTHFR 677TT Homozygote Jee-Young Lee, M.D., Manho Kim, M.D., Ph.D. Department of Neurology, College of Medicine, Seoul National University, Seoul, Korea Hyperhomocysteinemia associated with methylene terahydrofolate reductase (MTHFR) mutation can be a risk factor for idiopathic cerebral venous thrombosis. We describe the first case of MTHFR 677TT homozygote with cerebral venous thrombosis and livedo reticularis. A 45-year-old man presented with seizures and mottled-like skin lesions, that were aggravated by cold temperature. Hemorrhagic infarct in the right frontoparietal area with superior sagittal sinus thrombosis was observed. He had hyperhomocysteinemia, low plasma folate level, and MTHFR 677TT homozygote genotype, which might be associated with livedo reticularis and increase the risk for cerebral venous thrombosis. J Clin Neurol 2(2):137-140, 2006 Key Words : Livedo reticularis, Methylene tetrahydrofolate reductase, Cerebral venous thrombosis Hyperhomocysteinemia causes vascular endothelial venous infarct due to cerebral venous thrombosis. damage that result in atherosclerosis and ischemic strokes.1 It is also associated with prothrombotic state or venous thromboembolism2 including cerebral venous CASE REPORT thrombosis.3 Among the thrombophilic factors with hyperhomocysteinemia, methylene tetrahydrofolate reduc- A 45 year-old man was brought to the emergency tase (MTHFR) mutant (C677 → T, homozygote) with room with uncontrolled seizures. Two days ago, sudden low plasma folate concentration increases the risk for paresthesia in left arm developed, which progressed to cerebral venous thrombosis.4 MTHFR 677TT is thermo- tonic posturing and leftward head version, followed by labile and sensitive to temperature alteration.5 a generalized tonic clonic seizure. -

What Are Panic Attacks?
What are Panic Attacks? Understanding the body’s “Fight or Flight” response to a threat. We all have a built-in alarm system that turns on automatically to make sure we survive whatever danger triggers it. This alarm is a lot like a burglar alarm on a house; once it detects something that might be a threat, it automatically sets off several events. If someone were breaking into your house, you would want the alarm system to call the police immediately. You would also likely want to turn on lights and even sound an audible alarm to wake you and scare off the intruder. The brain’s alarm system sets off a series of events as well, commonly called the “fight or flight” response. The fight or flight response is meant to put us in a state of high alert so that we can fight off an enemy or flee to escape with our lives. This alarm makes us faster and stronger and more focused than we would normally be. Our ancestors may not have survived if the human body was not hardwired with the fight or flight response. The reaction is automatic. It helps us survive, and we do not have to think about it; it just happens. But because it is automatic, we do not get to choose which things will set it off. Sometimes, the fight or flight response can start firing if it thinks there is danger, even when there isn’t any real threat around. This is especially common in people who have been through a traumatic event. -

Livedoid Vasculopathy – Benefit of Intravenous Immunoglobulin in A
CASE REPORTS Ref: Ro J Rheumatol. 2021;30(1) DOI: 10.37897/RJR.2021.1.4 LIVEDOID VASCULOPATHY – BENEFIT OF INTRAVENOUS IMMUNOGLOBULIN IN A REFRACTORY CASE Stefan Cristian Dinescu1, Andreea Lili Barbulescu2, Paulina Lucia Ciurea1, Roxana Mihaela Dumitrascu3, Beatrice Andreea Chisalau3, Cristina Dorina Parvanescu3, Sineta Cristina Firulescu4, Florentin Ananu Vreju1 1 Department of Rheumatology, University of Medicine and Pharmacy, Craiova, Romania 2 Department of Pharmacology, University of Medicine and Pharmacy, Craiova, Romania 3Doctoral School, University of Medicine and Pharmacy, Craiova, Romania 4 Department of Rheumatology, Emergency County Hospital, Craiova, Romania Abstract Livedoid vasculopathy is a rare vascular disease which typically manifests as recurrent ulcerative lesions on the lower extremities. It is classified as a vasculopathy, not a true vasculitis, and defined as a vasooclusive syndrome, caused by non-inflammatory thrombosis of the upper and mid-dermal venulae. Main disorders associated with LV include thrombophilias, autoimmune diseases and neoplasia. A triad of clinical features is present in most patients and consist of livedo racemosa (less frequently livedo reticularis), ulcerations and atrophie blanche. Management generally relies on antiplatelet drugs, anticoagulants, vasodilators and fibrinolytic therapy. Some benefit has been observed with intravenous immunoglobulin, colchicine, hyperbaric oxygen, while glucocorticoids are efficient to a lesser extent. This case report highlights a refractory clinical form with no identifiable predisposing condition, which proved responsive only to intravenous immunoglobulin. Keywords: thrombosis, purpura, ulcer, intravenous immunoglobulins INTRODUCTION cal form with no identifiable predisposing condition, which proved responsive only to intravenous immu Livedoid vasculopathy (LV) is a rare vascular dis noglobulin. ease which typically manifests as recurrent ulcerative lesions on the lower extremities. -

Sneddon's Syndrome
DOI: 10.5272/jimab.14-1-2010.72 Journal of IMAB - Annual Proceeding (Scientific Papers) 2008, vol. 14, book 1 SNEDDON’S SYNDROME Valentin Valtchev1, Virginia Simeonova2 , Dimitar Gospodinov1, Ivelina Yordanova1, Valentina Dimitrova1, Verka Pavlova1, Emiliana Konova4, S. Popovska3 , Boyko Stamenov2 1Department of Dermatology and Venereology, 2Department of Neurology, 3Department of General and Clinical Pathology, 4Department of Immunology, Medical University – Pleven, Bulgaria ABSTRACT pressure and migraine for 10 years. She has been having Sneddon’s syndrome is usually characterized by the persistent cutaneous lesions on the upper and lower association of an ischemic cerebrovascular disease and a extremities and trunk for the last 20 years. widespread livedo reticularis. The incidence of Sneddon At physical examination, a slight elevation in syndrome is 4/1000 000. We present 42-year-old woman with pressure levels (150 x 80mm Hg), III degree obesity and livedo reticularis, recurrence ischaemic cerebrovascular slight edema of the lower limbs were found. The accidents, two repetitive miscarriages and positive anti-2GPi neurological examination revealed ataxic walk. Romberg antibodies. Skin biopsy specimens reveal inflammatory reflex was negative (-) and Babinski was positive (+) in changes of small- to medium-sized arteries and right. The ophthalmologic examination demonstrated an subendothelial proliferation and fibrosis. The diagnosis initial angiosclerosis. The dermatological examination Sneddon syndrome is confirmed by skin biopsy, and MR showed erythematous violaceous lesions with a reticular evidence. pattern, localized in the arms, trunk (Figure 1) thighs and We suggest that anti-2GPi antibodies may be knees (Figure 2, Figure 3). The following exams in the pathophysiologically related to the clinical manifestation laboratorial evaluation were normal or negative: blood count observed in some patients with Sneddon syndrome. -

Upper Limb Pain and Paresthesia in a Post-Stroke Patient Treated With
02 Brain Neurorehabil. 2018 Mar;11(1):e1 https://doi.org/10.12786/bn.2018.11.e1 pISSN 1976-8753·eISSN 2383-9910 Brain & NeuroRehabilitation Case Upper Limb Pain and Paresthesia in a Post-Stroke Patient Treated with Ultrasound-Guided Electrical Twitch-Obtaining Intramuscular Stimulation(ETOIMS) of Scalene Muscles Je Shik Nam, Yeo-Reum Choe, Seo Yeon Yoon, Tae Im Yi Received: Sep 1, 2017 Highlights Revised: Sep 29, 2017 Accepted: Oct 2, 2017 • Disputed thoracic outlet syndrome (TOS) can be one of the possible causes of post-stroke Correspondence to pain. Tae Im Yi • Ultrasound-guided electrical twitch-obtaining intramuscular stimulation can be used as a Departments of Rehabilitation Medicine, safe and minimally invasive technique for the treatment of the disputed TOS. Bundang Jesaeng General Hospital, • Large-scale randomized controlled studies are needed to confirm its therapeutic efficacy. 20 Seohyeon-ro 180 beon-gil, Bundang-gu, Seongnam 13590, Korea. E-mail: [email protected] Copyright © 2018. Korea Society for Neurorehabilitation i 02 Brain Neurorehabil. 2018 Mar;11(1):e1 https://doi.org/10.12786/bn.2018.11.e1 pISSN 1976-8753·eISSN 2383-9910 Brain & NeuroRehabilitation Case Upper Limb Pain and Paresthesia in a Post-Stroke Patient Treated with Ultrasound-Guided Electrical Twitch-Obtaining Intramuscular Stimulation(ETOIMS) of Scalene Muscles Je Shik Nam , Yeo-Reum Choe , Seo Yeon Yoon , Tae Im Yi Department of Rehabilitation Medicine, Bundang Jesaeng General Hospital, Seongnam, Korea Received: Sep 1, 2017 ABSTRACT Revised: Sep 29, 2017 Accepted: Oct 2, 2017 In post-stroke patients, the pain or paresthesia of the affected limb is common. -

Prognosis of a Case with Paresthesia Associated with Prolonged Touching of an Endodontic Paste to the Inferior Alveolar Nerve
J Clin Exp Dent. 2011;3(Suppl1):e377-81. Inferior alveolar nerve paresthesia. Journal section: Oral Surgery doi:10.4317/jced.3.e377 Publication Types: Case Report Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve M. Cemil Buyukkurt 1, Hakan Arslan 2, H. Sinan Topcuoglu 2, M. Melih Omezli 3 1 PhD, DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Sifa University, İzmir, Turkey 2 DDS. Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, Turkey 3 DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ataturk University, Erzurum, Turkey Correspondence: Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, 25240, Turkey E-mail address: [email protected] Received: 10/02/2011 Accepted: 08/05/2011 Buyukkurt MC, Arslan H, Topcuoglu HS, Omezli MM. Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve. J Clin Exp Dent. 2011;3(Suppl1):e377- 81. http://www.medicinaoral.com/odo/volumenes/v3iSuppl1/jcedv3iSu- ppl1p377.pdf Article Number: 50506 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Abstract Paresthesia is described as an abnormal sensation, such as burning, pricking, tickling, tingling, formication or num- bness. Several conditions can cause paresthesia. This article presents a case of paresthesia caused by the extrusion of endodontic paste (Endomethasone®) into the mandibular canal. The clinical manifestations comprised the numb- ness on the right side of the mandible and right lower lip, appearing after endodontic treatment. -

Small Animal
Small Animal Sampler Separation Distress Syndrome From Blackwell’s Five-Minute Veterinary Consult – Canine and Feline, Sixth Edition. by Deborah F. Horwitz Chapter 236: Vomiting From The Feline Patient, Fifth Edition. Edited by Gary D. Norsworthy. Chapter 12: Pharmacologic and Clinical Principles of Adjunct Analgesia From Analgesia and Anesthesia for the Ill or Injured Dog and Cat, First Edition. by Karol A. Mathews, Melissa Sinclair, Andrea M. Steele, and Tamara Grubb. 1208 Blackwell’s Five-Minute Veterinary Consult Separation Distress Syndrome commonly reported. Destruction targets windows and doors and/or owner possessions. Other signs include behavioral depression, BASICS anorexia,r drooling, hiding, shaking, panting, DIAGNOSIS DEFINITION pacing, attempts to prevent owner departure, DIFFERENTIAL DIAGNOSIS A distress response of dogs (occasionally cats) and self-trauma from lick lesions. Diarrhea Vocalization: response to outdoor separated from the person or persons to and vomiting are occasionally noted. Signs influences,r territorial displays, play with other whom they are most attached, usually their of strong pet-owner attachment may ber pets in the home or fears. Destructive owner(s). The separation may be real (the present: excessive attention-seeking behaviors behaviors: occur both whenr the owner is owner is gone) or perceived (the pet is just and following behaviors but not necessary for present and absent (e.g., territorial destructive separated from the owner). In other cases the diagnosis. Frequently owners report displays at windows and doors; destruction pet may be distressed because some excessive, excited,r and prolonged greeting due to fear-producing stimuli such as noises fear-inducing event has occurred while home behavior upon return. -

Post-Burn Pruritus
International Journal of Molecular Sciences Review Post-Burn Pruritus 1, 1, 1 1 2 Bo Young Chung y, Han Bi Kim y, Min Je Jung , Seok Young Kang , In-Suk Kwak , Chun Wook Park 1 and Hye One Kim 1,* 1 Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University, Seoul 07441, Korea; [email protected] (B.Y.C.); [email protected] (H.B.K.); [email protected] (M.J.J.); [email protected] (S.Y.K.); [email protected] (C.W.P.) 2 Department of Anesthesiology and Pain Medicine, Burn Center, Hangang Sacred Heart Hospital, Hallym University, Seoul 07247, Korea; [email protected] * Correspondence: [email protected]; Tel.: 82-2-829-5221 Co-first authors: [email protected] (B.Y.C.); [email protected] (H.B.K). y Received: 8 April 2020; Accepted: 21 May 2020; Published: 29 May 2020 Abstract: Post-burn pruritus is the pruritus that occurs after burn during the rehabilitation and healing process of burn wounds. The post-burn pruritus is a common and serious complication of burn injury, which severely lowers the quality of life of the patient. Many potential treatments are available for pruritus but there is no consensus of the best single treatment yet. The precise mechanism of post-burn pruritus has not been elucidated, but it appears to have pruritogenic and neuropathic aspects. Clinically, post-burn pruritus tends to be intractable to conventional treatment but rather responds to neuroleptic agents, such as gabapentin and pregabalin. During wound healing, various neuropeptides secreted from the nerves of the skin control epidermal and vascular proliferation and connective tissue cells. -
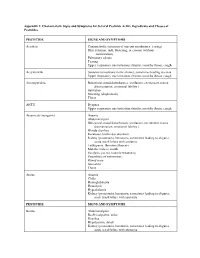
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides PESTICIDE SIGNS AND SYMPTOMS Acrolein Conjunctivitis (irritation of mucous membranes, tearing) Skin irritation, rash, blistering, or erosion (without sensitization) Pulmonary edema Tearing Upper respiratory tract irritation: rhinitis, scratchy throat, cough Acrylonitrile Seizures/convulsions (tonic-clonic), sometimes leading to coma Upper respiratory tract irritation: rhinitis, scratchy throat, cough Aminopyridine Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Salivation Sweating (diaphoresis) Thirst ANTU Dyspnea Upper respiratory tract irritation: rhinitis, scratchy throat, cough Arsenicals (inorganic) Anemia Abdominal pain Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Bloody diarrhea Keratoses, brown discoloration Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Leukopenia, thrombocytopenia Metallic taste in mouth Paralysis, paresis (muscle weakness) Paresthesia of extremities Runny nose Stomatitis Thirst Arsine Anemia Chills Hemoglobinuria Hemolysis Hyperkalemia Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia PESTICIDE SIGNS AND SYMPTOMS Borate Abdominal pain Beefy red palms, soles Diarrhea Hypotension, shock Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Nervous system depression -

Painless, Atraumatic, Isolated Lateral Compartment Syndrome of the Leg: an Unusual Triad of Atypical Findings
A Case Report & Literature Review Painless, Atraumatic, Isolated Lateral Compartment Syndrome of the Leg: An Unusual Triad of Atypical Findings Luke S. Oh, MD, Paul B. Lewis, MD, Mark L. Prasarn, MD, Dean G. Lorich, MD, and David L. Helfet, MD cute compartment syndrome is a potentially Abstract devastating condition in which the pressure Compartment syndrome can be a devastating complica- within an osseofascial compartment rises to tion with significant morbidity when not recognized or a level that decreases the perfusion gradient treated expediently. Among the classic pentad of signs Aacross tissue capillary beds, leading to cellular anoxia, and symptoms associated with compartment syndrome, muscle ischemia, and necrosis. Diagnosis is primar- pain that is out of proportion to the injury is often cited ily clinical, supplemented by compartment pressure as the earliest and most sensitive. We present a case report of an atypical presentation measurements. The clinical diagnosis of acute com- of compartment syndrome of the leg in which a patient partment syndrome is typically made on the basis of taking lithium for bipolar disorder did not report pain out a classically known pentad of symptoms: pain, pallor, of proportion to the injury mechanism. Lithium has been paresthesia, paralysis, and pulselessness.1,2 Among the implicated in altering pain perception and increasing the “5 P’s” of compartment syndrome, pallor, paralysis, tolerance and threshold for pain, but this has not been and pulselessness are considered to be late findings.1,2 widely reported in the orthopedic literature. It is well established that pain with passive stretch and In addition to compartment syndrome that was painless, pain that is out of proportion to the injury are the earli- the patient presented with 2 additional atypical findings. -

Capecitabine-Induced Lichenoid Drug Eruption: a Case Report
Volume 23 Number 2 | February 2017 Dermatology Online Journal || Case Report DOJ 23 (2): 2 Capecitabine-induced lichenoid drug eruption: a case report Jeff R. Gehlhausen PhD1, Matthew B. Strausburg MD2, Mouhammad Aouthmany MD2, Terrence M. Katona3, Matthew J. Turner MD, PhD3 Affiliations: 1Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, Indiana, 2Department of Dermatology, Indiana University School of Medicine, Indianapolis, Indiana, 3Richard L. Roudebush VA Medical Center, Indianapolis, Indiana Corresponding Author: Matthew J. Turner, Address: 545 Barnhill Dr (EH139); Indianapolis, IN 46202, Telephone: 317-274-7740, Fax: 317- 274-3700, Email: [email protected] Abstract DNA synthesis through inhibition of the enzyme thymidylate synthetase, thereby sensitizing rapidly Capecitabine is a 5-fluorouracil based growing cells to cell death. Systemic use of 5-FU was chemotherapeutic drug widely used in the treatment long ago shown to result in skin photosensitivity, as of solid tumors, especially colorectal and breast. Some well as an inflammatory reaction in actinic keratoses of the most common side effects of capecitabine are [1,2]. On this basis, it has been suggested that the cutaneous in nature, including hand-foot syndrome anti-tumor activity observed by topical 5-FU on (palmar-plantar erythrodysesthesia). Several reports in actinic keratoses is in part related to the production the literature link capecitabine use with photosensitive of an immunologic reaction in tandem with its lichenoid eruptions. Herein, we present a case of anti-metabolite function [3]. Prodrugs of 5-FU (e.g. capecitabine-induced lichenoid eruption in an elderly capecitabine) have also been reported to cause female with metastatic breast cancer and discuss our a variety of cutaneous eruptions [4-7] (Table 1).