The Innervation of the Upper Urinary Tract J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

Kidney in an Effort to Aid Health Information Management Coding Professionals for ICD-10, the Following Anatomy Tip Is Provided with an Educational Intent
Anatomy Tip Kidney In an effort to aid Health Information Management Coding Professionals for ICD-10, the following anatomy tip is provided with an educational intent. The kidneys are the main part of the urinary system and are a pair of organs; right and left; located in the back of the abdomen. Each kidney is about 4 or 5 inches long. • The kidneys are surrounded by three layers of tissue: • The renal fascia is a thin, outer layer of fibrous connective tissue that surrounds each kidney (and the attached adrenal gland) and fastens it to surrounding structures. • The adipose capsule is a middle layer of adipose (fat) tissue that cushions the kidneys. • The renal capsule is an inner fibrous membrane that prevents the entrance of infections. • There are three major regions inside the kidney: • The renal cortex borders the convex side. • The renal medulla lies adjacent to the renal cortex. It consists of striated, cone-shaped regions called renal pyramids (medullary pyramids), whose peaks, called renal papillae, face inward. The unstriated regions between the renal pyramids are called renal columns. • The renal sinus is a cavity that lies adjacent to the renal medulla. The other side of the renal sinus, bordering the concave surface of the kidney, opens to the outside through the renal hilus. The ureter, nerves, and blood and lymphatic vessels enter the kidney on the concave surface through the renal hilus. The renal sinus houses the renal pelvis, a funnel-shaped structure that merges with the ureter. • All the blood in our bodies passes through the kidneys several times a day. -

12 Renal Sinus Neoplasms
Renal Sinus Neoplasms 187 12 Renal Sinus Neoplasms Sung Eun Rha and Jae Young Byun CONTENTS fibers of the autonomic nervous system, and varying quantities of fibrous tissue (Amis 2000; Amis and 12.1 Introduction 187 Cronan 1988; Davidson et al. 1999; Zagoria and 12.2 Imaging Modalities for Renal Sinus Tumors 188 Tung 12.3 Epithelial Tumors of the Renal Pelvis 189 1997b). Of these constituents, fat is the largest 12.3.1 Transitional Cell Carcinoma 189 single component of the renal sinus and is read- 12.3.2 Squamous Cell Carcinoma 190 ily seen with ultrasound (US), computed tomogra- 12.4 Mesenchymal Tumors of the Renal Sinus 192 phy (CT), and magnetic resonance (MR) imaging. 12.4.1 Leiomyosarcoma 193 The quantity of fat in the renal sinus normally and 12.4.2 Hemangiopericytoma 193 gradually increases with age and obesity (Fig. 12.3; 12.4.3 Differential Diagnosis 194 Zagoria Tung 12.5 Renal Parenchymal Tumors Projecting into and 1997b). Observation of renal the Renal Sinus 195 sinus fat is important for detecting small tumors 12.5.1 Renal Cell Carcinoma 195 in that area, as well as for determining the exact 12.5.2 Multilocular Cystic Nephroma 195 tumor staging. 12.6 Retroperitoneal Tumors Extending to the Renal Sinus 199 Renal sinus involvement of tumors is significant 12.6.1 Lymphoma 199 because the renal sinus contains numerous lymphat- 12.6.2 Metastasis 200 ics and veins that may permit dissemination of a 12.7 Conclusion 200 tumor otherwise regarded as renal limited. Because References 200 there is no fibrous capsule separating the renal cortex of the columns of Bertin from the renal sinus fat, a renal tumor may continue unrestricted into the sinus fat, which is rich in veins and lymphatics 12.1 (Bonsib et al. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -

Renal Ultrasound (Basic Principles) BMUS Study Day
Renal Ultrasound (Basic Principles) BMUS Study Day Rosie Conlon Clinical Specialist Sonographer NATIONAL REHABILITATION HOSPITAL Dun laoghaire, Dublin Sat 15th October 2016 WHY? “Bones can break, muscles can atrophy, glands can loaf about and even the brain can sleep without immediate danger to survival. BUT when the kidneys fail…. Neither bone, muscle gland nor brain could carry on.” Homer William Smith, “The Evolution of the Kidney”, Lectures on the Kidney (1943). Renal Ultrasound (Basic Principles) and BMUS Study Case PREPARATION • 500mls 1 hour before, avoiding • Operator Dependant micturition. • Catheter clamped 1.5 hours • Real Time before. • Fluids by PEG 1.5 hours before. • Reproducible • Non-invasive • Inspiration WHAT? - PROTOCOL • Both Kidneys • Urinary Bladder • +/- Residual Volume • Pelvic Surveillance • Aorta • Local protocol pertinent to the population • Full bladder The most important step in diagnosis is realising that it might exist. Renal Ultrasound (Basic Principles) and BMUS Study Case RIGHT KIDNEY-TECHNIQUE • A 3.5-5 MHz probe is typically used to scan the kidney. For the right kidney, have the patient lie supine and place the probe in the right lower intercostal space in the midaxillary line. Use the liver as your “acoustic window” and aim the probe slightly posteriorly (toward the kidney). Gently rock the probe (up and down or side to side) to scan the entire kidney. If needed, you can have the patient inspire or exhale, which allows for subtle movement of the kidney. • Obtain longitudinal (long axis) and transverse (short axis) views. Renal Ultrasound (Basic Principles) and BMUS Study Case ANATOMY Hepatic Veins Spleen Celiac axis Liver SMA Left Right Renal artery kidney kidney Renal vein Renal Ultrasound (Basic Principles) and BMUS Study Case NORMAL Renal Ultrasound (Basic Principles) and BMUS Study Case LEFT KIDNEY-TECHNIQUE • For the left kidney have the patient lie supine or in the right lateral decubitus position. -

Anatomy of the Kidney
Anatomy of the kidney Renal block-Anatomy-Lecture 1 Editing file Color guide : Only in boys slides in Green Objectives Only in girls slides in Purple important in Red Notes in Grey By the end of this course you should be able to discuss : ● Components of the urinary system ● Kidney : 1. Shape & Position 2. Surface anatomy 3. External features 4. Hilum & its contents 5. Relation 6. Internal features 7. Blood supply 8. Lymph drainage 9. Nerve supply Introduction 3 ● Every day, each kidney filters liters (around 150 L per day ) of fluid from the bloodstream. ● Although the lungs and the skin also play roles in excretion, The kidneys bear the major responsibility for eliminating nitrogenous (nitrogen-containing) wastes, toxins, and drugs from the body. Excretes most of the Maintain acid-base waste products of balance of the blood. metabolism. By Erythropoietin Controls hormone stimulates Function of water & electrolyte bone marrow for RBCs kidney balance of the body. formation. Converts By Rennin vitamin D to its enzyme regulates active form. the blood pressure. The kidney : ● Kidneys are reddish brown in color. 4 ● Lie behind the peritoneum (retroperitoneal) on the posterior abdominal wall on either side of the vertebral column. ● They are largely under cover of the costal margin. kidney lies between T12-L3 ● With contraction of the diaphragm (during inspiration) the kidney moves downward as much as 2.5 cm. T12 Comparison between : L3 Right kidney Left kidney Anterior view lies slightly lower than the left due Location to the large size of the right lobe of Upper than the right the liver. -
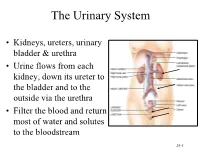
Urinary System
The Urinary System • Kidneys, ureters, urinary bladder & urethra • Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra • Filter the blood and return most of water and solutes to the bloodstream 26-1 Overview of Kidney Functions • Regulation of blood ionic composition – Na+, K+, Ca+2, Cl- and phosphate ions • Regulation of blood pH, osmolarity & glucose • Regulation of blood volume – conserving or eliminating water • Regulation of blood pressure • Release of erythropoietin & calcitriol • Excretion of wastes & foreign substances 26-2 Internal Anatomy of the Kidneys • Parenchyma of kidney – renal cortex = superficial layer of kidney – renal medulla • inner portion consisting of 8-18 cone-shaped renal pyramids separated by renal columns • renal papilla point toward center of kidney • Drainage system fills renal sinus cavity – cuplike structure (minor calyces) collect urine from the papillary ducts of the papilla – minor & major calyces empty into the renal pelvis which empties into the ureter 26-3 Internal Anatomy of Kidney Human Kidney 26-5 Blood & Nerve Supply of Kidney • Abundantly supplied with blood vessels – receive 25% of resting cardiac output via renal arteries • Functions of different capillary beds – glomerular capillaries where filtration of blood occurs – peritubular capillaries that carry away reabsorbed substances from filtrate (renal cortex) – vasa recta supplies nutrients to medulla • Sympathetic vasomotor nerves regulate blood flow by altering arterioles 26-6 Blood Vessels around -

The Urinary System Consists of Kidneys, Ureters, Urinary Bladder
Anatomy Lecture Notes Chapter 23 the urinary system consists of kidneys, ureters, urinary bladder, and urethra the function of the kidneys is NOT "to make urine" the kidneys: 1) regulate water balance 2) regular ECF electrolyte levels (Na, K, Ca) 3) eliminate some metabolic wastes urine is a by-product of these functions A. kidneys 1. located against posterior abdominal wall (retroperitoneal) T11 or T12 to L3 right kidney lower than left kidney 2. surrounded by a. pararenal fat (posterior only) b. renal fascia c. adipose capsule - perirenal fat d. renal capsule - dense c.t. covering surface of kidney e. parietal peritoneum Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 3. layers a. cortex - contains renal corpuscles and extends inwards as renal columns b. medulla - consists of renal pyramids which consist mostly of collecting ducts papilla - apex of renal pyramid; where collecting ducts drain into calyx 4. cavities and associated structures a. renal sinus - space in medial part of kidney; contains renal pelvis b. renal pelvis - expanded superior part of ureter minor calyx collects urine from one renal papilla major calyx formed by junction of 2 or more minor calyces renal pelvis formed by junction of all major calyces Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 5. renal hilum - medial indentation; where ureter leaves kidney 6. blood flow through the kidney - renal fraction = 20% of cardiac output aorta renal artery segmental arteries lobar arteries interlobar arteries arcuate arteries cortical radiate (interlobular) arteries afferent arterioles glomerular capillaries (glomerulus) efferent arteriole peritubular capillaries and vasa recta cortical radiate (interlobular) veins arcuate veins interlobar veins renal vein inferior vena cava Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 7. -

ANATOMY Urinary System
ANATOMY Urinary system 1st Prof Pharm.D Urinary system Composition of urinary system: The urinary system consists of ❑ two kidneys, ❑two ureters, ❑the urinary bladder, ❑the urethra. Kidneys Kidneys are paired, reddish, bean–shaped organs responsible for formation of urine by blood filtration Location Kidneys are positioned against the posterior wall of the abdominal cavity (retroperitoneal) between the levels of the twelfth thoracic and the third lumbar vertebrae The right kidney is usually 1.5 to 2.0 cm lower than the left because of the large area occupied by the liver on the right side. External anatomy of kidneys Each adult kidney is a bean-shaped organ about 11.25 cm (4 in.) long, 5.5 to 7.7 cm (2–3 in.) wide, and 2.5 cm (1 in.) thick. Each kidney is embedded in a fatty fibrous pouch consisting of three layers: ❑ The renal capsule - layer of dense fibrous connective tissue surrounding each kidney, protects kidney from trauma and infection and maintain its shape ❑ Renal adipose capsule – layer of adipose tissue surrounding the renal capsule functions as a shock absorber, cushioning the kidneys against mechanical shock. ❑ Renal fascia - thin layer of connective tissue surrounding the adipose tissue, anchors the kidney to the posterior abdominal wall * The lateral border of each kidney is convex, whereas the medial border is strongly concave * On the medial border is hilum, a depression through which renal artery, vein and nerves enter and leave kidney * The superior border of each kidney is capped by the adrenal gland Internal anatomy of kidneys The coronal section of the kidney show two distinct regions and a cavity. -

Aandp2ch23lecture.Pdf
Chapter 23 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Urinary system rids the body of waste products • Kidneys also play important roles in blood volume, pressure, and composition • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filter blood plasma, excrete toxic wastes • Regulate blood volume, pressure, and osmolarity • Regulate electrolytes and acid-base balance • Secrete erythropoietin, which stimulates the production of red blood cells • Help regulate calcium levels by participating in calcitriol synthesis • Clear hormones from blood • Detoxify free radicals • In starvation, they synthesize glucose from amino acids 23-5 Retroperitoneal Position of the Kidney Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. -

Genitourinary Grossing Guidelines Specimen Type
Genitourinary Grossing Guidelines Specimen Type: NEPHROURETERECTOMY Procedure: 1. Review radiology and prior path reports for tumor location. 2. Inspect and palpate ureter and renal pelvis for tumor, palpate for hilar lymph nodes, and perinephric fat for adrenal. 3. Ink surface of kidney (Gerota’s fascia or perinephric fat) and ureter. a. Can differentially ink the ureter with three colors- proximal, mid, distal b. Usually a bladder cuff is present (a small portion of bladder surrounding the ureteral orifice), ink peripheral margins of bladder cuff in a different color. 4. Measure the overall size of kidney with perinephric fat, length and diameter of ureter, diameter and thickness of bladder cuff. 5. Remove renal vein and artery margins and place in a cassette. Bladder cuff margin --- amputate 5-7 mm in length including a small portion of distal ureter, radially section (like a cervical cone), and submit sections in 2-3 cassettes. If no bladder cuff, submit the distal ureter margin en face. 6. Place a probe into ureter, and extend it into renal pelvis. Open ureter along its length. Inspect ureteral mucosa for tumor and erythematous flat lesions. 7. At renal hilum, push one probe through renal pelvicalyceal system and push through parenchyma of superior pole of kidney. 8. Place second probe in renal pelvicalyceal system and push through parenchyma of inferior pole of kidney. 9. Using probes as guides, divide kidney in two complete halves, cutting through the renal pelvis. Completely open pelvis, calyces, and renal veins. 10. Measure kidney, and adrenal (if present). 11. Examine renal pelvis and ureter for tumor(s). -

NEPHRECTOMY for Tumor
Genitourinary Grossing Guidelines Specimen Type: NEPHRECTOMY For Tumor Page | 1 Genitourinary Grossing Guidelines Procedure: 1. Weigh and measure overall dimensions of specimen. Ink the surface of Gerota’s fascia or perinephric fat, either the entire surface or the surface overlying a palpable tumor. 2. Inspect perinephric fat for adrenal, tumor extension; palpate for hilar lymph nodes. 3. Locate ureter (often with a staple or clip at the distal end). Measure the length and diameter of ureter. Locate renal arteries and vein, look for tumor thrombus in the renal vein. 4. Remove vascular and ureteral margins and place in cassette, en face. 5. Place a probe into ureter, and extend it into renal pelvis. Open ureter along its length. Examine ureteral mucosa. 6. At renal hilum, push one probe through renal pelvicalyceal system and push through parenchyma of superior pole of kidney. 7. Place second probe in renal pelvicalyceal system and push through parenchyma of inferior pole of kidney. 8. Using 2 probes as guides, divide kidney in two complete halves, cutting through the renal pelvis. Completely open pelvis, calyces, and renal veins. 9. Measure kidney. 10. Look for renal sinus invasion, renal vein invasion, and capsular invasion by tumor. Measure and describe adrenal (? tumor involvement) if present. 11. Photograph half of the specimen. 12. Describe tumor: single/multiple lesions, dimensions, demarcation, color, texture, hemorrhage/necrosis/cystic degeneration, extension into renal sinus/into renal vein/through capsular surface, areas of sarcomatoid differentiation. 13. After taking sections of tumor, bread loaf the uninvolved kidney in 1 cm interval, look for additional lesions, describe uninvolved kidney: external surface, cortex, medulla, and pelvis.