2010286569 C1 (19) Australian Patent 0Ffice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Direct Preparation of Some Organolithium Compounds from Lithium and RX Compounds Katashi Oita Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1955 Direct preparation of some organolithium compounds from lithium and RX compounds Katashi Oita Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Oita, Katashi, "Direct preparation of some organolithium compounds from lithium and RX compounds " (1955). Retrospective Theses and Dissertations. 14262. https://lib.dr.iastate.edu/rtd/14262 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -

United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill Et Al
US005.945382A United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill et al. (45) Date of Patent: *Aug. 31, 1999 54 FUNGICIDAL ARYLPYRAZOLES 2300173 12/1990 Japan. 2224208 5/1990 United Kingdom. 75 Inventors: Richard Cantegril, Lyons; Denis Croisat, Paris; Philippe Desbordes, OTHER PUBLICATIONS Lyons, Francois Guigues, English translation of JP 2-300173, 1990. Rillieux-la-Pape; Jacques Mortier, La English translation of JP 59–53468, 1984. Bouéxier; Raymond Peignier, Caluire; English translation of JP 3-93774, 1991. Jean Pierre Vors, Lyons, all of France Miura et al., (CA 1.14:164226), 1991. Miura et al., (CA 115:92260), 1991. 73 Assignee: Rhone-Poulenc Agrochimie, Lyons, Chemical Abstracts, vol. 108, No. 23, 1986, abstract No. France 204577b. CAS Registry Handbook, No. section, RN=114913-44-9, * Notice: This patent is subject to a terminal dis 114486-01-0, 99067-15-9, 113140-19-5, 73227-97-1, claimer. 27069-17-6, 18099-21–3, 17978-27-7, 1988. 21 Appl. No.: 08/325,283 Hattori et al., CA 68:68981 (1968), Registry No. 17978–25–5, 17978-26-6, 17978-27-7 and 18099–21-3. 22 PCT Filed: Apr. 26, 1993 Hattori et al., CA 68:68982 (1968), Registry No. 17978-28-8. 86 PCT No.: PCT/FR93/00403 Janssen et al., CA 78: 159514 (1973), Registry No. S371 Date: Dec. 22, 1994 38858-97-8 and 38859-02-8. Chang et al., CA 92:146667 (1980), Registry No. S 102(e) Date: Dec. 22, 1994 73227 91-1. Berenyi et al., CA 94:156963 (1981), Registry No. -
ESSEE in Which R1 and R2 Are the Same Or Different And
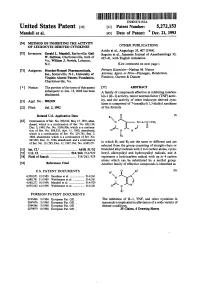
USOO5272153A United States Patent (19) 11 Patent Number: 5,272,153 Mandell et al. 45 Date of Patent: Dec. 21, 1993 (54)54 OFMETH E. NHIBITINSi6. CTVTY OTHER PUBLICATIONS Avido et al., Angiology 35, 407 (1984). 75 Inventors: St. Mel ES Suguira et al., Japanese Journal of Anesthesiology 32, va.8 William,Wan, CharlotteSVlie, Novici, Lebanon, O 435-41,35-41, with English translationtranslation. N.J. (List continued on next page.) 73) Assignees: Hoechst-Roussel Pharmaceuticals, Primary Examiner-Nathan M. Nutter Inc., Somerville, N.J.; University of Attorney, Agent, or Firm-Finnegan, Henderson, Virginia Alumni Patents Foundation, Farabow, Garrett & Dunner Charlottesville, Va. (*) Notice: The portion of the term of this patent 57) ABSTRACT E. to Jun 15, 2008 has been A family of compounds effective in inhibiting interleu SC. kin-1 (IL-1) activity, tumor necrosis factor (TNF) activ (21) Appl. No.: 908,929 ity, and the activity of other leukocyte derived cyto s kines is comprised of 7-(oxoalkyl) 1,3-dialkyl xanthines (22 Filed: Jul. 2, 1992 of the formula Related U.S. Application Data R (I) 63 Continuation of Ser. No. 700,522, May 15, 1991, aban- Y N-A-C-CH3 doned, which is a continuation of Ser. No. 622,138, Dec. 5, 1990, Pat. No. 5,096,906, which is a continua- al- 6 tion of Ser. No. 508,535, Apr. 11, 1990, abandoned, O N N which is a continuation of Ser. No. 239,761, Sep. 2, 1988, abandoned, which is a continuation of ser. No. R2 ESSEE selectedin which from R1 and the R2group are consistingthe same orof different straight-chain and are or 51) int. -

OMLI041 GHS US English US
OMLI041 - METHYLLITHIUM, 3M in diethoxymethane METHYLLITHIUM, 3M in diethoxymethane Safety Data Sheet OMLI041 Date of issue: 10/04/2017 Revision date: 05/24/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product name : METHYLLITHIUM, 3M in diethoxymethane Product code : OMLI041 Product form : Mixture Physical state : Liquid Formula : CH3Li Synonyms : LITHIUM METHIDE Chemical family : METAL ALKYL 1.2. Recommended use and restrictions on use Recommended use : Chemical intermediate 1.3. Supplier GELEST, INC. 11 East Steel Road Morrisville, PA 19067 USA T 215-547-1015 - F 215-547-2484 - (M-F): 8:00 AM - 5:30 PM EST [email protected] - www.gelest.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids Category 2 H225 Highly flammable liquid and vapor Pyrophoric liquids Category 1 H250 Catches fire spontaneously if exposed to air Substances and mixtures which in contact with water emit flammable H260 In contact with water releases flammable gases which may ignite gases Category 1 spontaneously Skin corrosion/irritation Category 1B H314 Causes severe skin burns and eye damage Serious eye damage/eye irritation Category 1 H318 Causes serious eye damage Specific target organ toxicity (single exposure) Category 3 H335 May cause respiratory irritation Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H225 - Highly flammable liquid and vapor H250 - Catches fire spontaneously if exposed to air H260 - In contact with water releases flammable gases which may ignite spontaneously H314 - Causes severe skin burns and eye damage H318 - Causes serious eye damage H335 - May cause respiratory irritation Precautionary statements (GHS US) : P280 - Wear protective gloves/protective clothing/eye protection/face protection. -

Organic Seminar Abstracts
L I B RA FLY OF THE U N IVERSITY OF 1LLI NOIS Q.54-1 Ii£s 1964/65 ,t.l P Return this book on or before the Latest Date stamped below. Theft, mutilation, and underlining of books are reasons for disciplinary action and may result in dismissal from the University. University of Illinois Library OCfcfcsrm L161— O-1096 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemi1964651univ s ORGANIC SEMINAR ABSTRACTS 196^-65 Semester I Department of Chemistry and Chemical Engineering University of Illinois ' / SEMINAR TOPICS I Semester I96U-I965 f"/ ( Orientation in Sodium and Potassium Metalations of Aromatic Compounds Earl G. Alley 1 Structure of Cyclopropane Virgil Weiss 9 Vinylidenes and Vinylidenecarbenes Joseph C. Catlin 17 Diazene Intermediates James A. Bonham 25 Perfluoroalkyl and Polyfluoroalkyl Carbanions J. David Angerer 3^ Free Radical Additions to Allenes Raymond Feldt 43 The Structure and Biosynthesis of Quassin Richard A. Larson 52 The Decomposition of Perester Compounds Thomas Sharpe 61 The Reaction of Di-t -Butyl Peroxide with Simple Alkyl, Benzyl and Cyclic Ethers R. L. Keener 70 Rearrangements and Solvolysis in Some Allylic Systems Jack Timberlake 79 Longifolene QQ Michael A. Lintner 00 Hydrogenation ! Homogeneous Catalytic Robert Y. Ning 9° c c Total Synthesis of ( -) -Emetime R. Lambert 1* Paracyclophanes Ping C. Huang 112 Mechanism of the Thermal Rearrangement of Cyclopropane George Su 121 Some Recent Studies of the Photochemistry of Cross -Conjugated Cyclohexadienones Elizabeth McLeister 130 The Hammett Acidity Function R. P. Quirk 139 Homoaromaticity Roger A. -

Silyl Ketone Chemistry. Preparation and Reactions of Silyl Allenol Ethers. Diels-Alder Reactions of Siloxy Vinylallenes Leading to Sesquiterpenes2
J. Am. Chem. SOC.1986, 108, 7791-7800 7791 pyrany1oxy)dodecanoic acid, 1.38 1 g (3.15 mmol) of GPC-CdCIz, 0.854 product mixture was then filtered and concentrated under reduced g (7.0 mmol) of 4-(dimethylamino)pyridine, and 1.648 g (8.0 mmol) of pressure. The residue was dissolved in 5 mL of solvent B and passed dicyclohexylcarbodiimide was suspended in 15 mL of dry dichloro- through a 1.2 X 1.5 cm AG MP-50 cation-exchange column in order to methane and stirred under nitrogen in the dark for 40 h. After removal remove 4-(dimethylamino)pyridine. The filtrate was concentrated under of solvent in vacuo, the residue was dissolved in 50 mL of CH30H/H20 reduced pressure, dissolved in a minimum volume of absolute ethanol, (95/5, v/v) and stirred in the presence of 8.0 g of AG MP-50 (23 OC, and then concentrated again. Chromatographic purification of the res- 2 h) to allow for complete deprotection of the hydroxyl groups (monitored idue on a silica gel column (0.9 X 6 cm), eluting first with solvent A and by thin-layer chromatography)." The resin was then removed by fil- then with solvent C (compound 1 elutes on silica as a single yellow band), tration and the solution concentrated under reduced pressure. The crude afforded, after drying [IO h, 22 OC (0.05 mm)], 0.055 g (90%) of 1 as product (2.75 g). obtained after drying [12 h, 23 OC (0.05 mm)], was a yellow solid: R 0.45 (solvent C); IR (KBr) ucz0 1732, uN(cH3)3 970, then subjected to chromatographic purification by using a 30-g (4 X 4 1050, 1090cm-'; I' H NMR (CDCI,) 6 1.25 (s 28 H, CH2), 1.40-2.05 (m, cm) silica gel column, eluting with solvents A and C, to yield 0.990 g 20 H, lipoic-CH,, CH2CH20,CH2CH,C02), 2.3 (t. -

Polyorganosiloxanes: Molecular Nanoparticles, Nanocomposites and Interfaces
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses November 2017 POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES Daniel H. Flagg University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Materials Chemistry Commons, Polymer and Organic Materials Commons, and the Polymer Chemistry Commons Recommended Citation Flagg, Daniel H., "POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES" (2017). Doctoral Dissertations. 1080. https://doi.org/10.7275/10575940.0 https://scholarworks.umass.edu/dissertations_2/1080 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the degree requirements for the degree of DOCTOR OF PHILOSOPHY September 2017 Polymer Science and Engineering © Copyright by Daniel H. Flagg 2017 All Rights Reserved POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Approved as to style and content by: Thomas J. McCarthy, Chair E. Bryan Coughlin, Member John Klier, Member E. Bryan Coughlin, Head, PS&E To John Null ACKNOWLEDGEMENTS There are countless individuals that I need to thank and acknowledge for getting me to where I am today. I could not have done it alone and would be a much different person if it were not for the support of my advisors, friends and family. -

Preparations, Solution Composition, and Reactions of Complex Metal Hydrides and Ate Complexes of Zinc, Aluminum, and Copper a Th
PREPARATIONS, SOLUTION COMPOSITION, AND REACTIONS OF COMPLEX METAL HYDRIDES AND ATE COMPLEXES OF ZINC, ALUMINUM, AND COPPER A THESIS Presented to The Faculty of the Division of Graduate Studies By John Joseph Watkins In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry Georgia Institute of Technology April, 1977 PREPARATIONS, SOLUTION COMPOSITION, AND REACTIONS OF COMPLEX METAL HYDRIDES AND ATE COMPLEXES OF ZINC, ALUMINUM, AND COPPER Approved: Erlin^rbrovenstein, Jr., Chairman H. 0. House E. C. Ashby Date approved by Chairman £~^3c>l~l~f ii ACKNOWLEDGMENTS Many individuals and organizations have contributed to the successful completion of this thesis. The following acknowledgments are not complete, but I hope I have expressed my gratitude to the people and organizations upon whom I depended the most. The School of Chemistry supported my first three years of work by the award of an NSF fellowship. My last year of work was generously supported by the St. Regis Paper Company, who graciously gave me leave of absence with salary so that the requirements for this thesis could be completed. This stipend and tuition support of my work freed me to concentrate on research without the financial difficulties encountered by many graduate students. All the faculty and staff of the School of Chemistry supported my research. I particularly would like to recognize Professor W. M. Spicer, Professor J. A. Bertrand, Professor C. L. Liotta, Mr. Gerald O'Brien, and Mr. D. E. Lillie. Post-doctoral assistants and fellow graduate students who contributed to my experience at the Georgia Insti tute of Technology include Dr. -

Reintroduction of Lithium Into Recycled Electrode
(19) TZZ Z_T (11) EP 2 248 220 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: H01M 10/052 (2010.01) H01M 10/42 (2006.01) 04.11.2015 Bulletin 2015/45 H01M 10/54 (2006.01) H01M 4/485 (2010.01) H01M 4/505 (2010.01) H01M 4/525 (2010.01) (2010.01) (21) Application number: 09713108.0 H01M 4/58 (22) Date of filing: 20.02.2009 (86) International application number: PCT/US2009/034779 (87) International publication number: WO 2009/105713 (27.08.2009 Gazette 2009/35) (54) REINTRODUCTION OF LITHIUM INTO RECYCLED ELECTRODE MATERIALS FOR BATTERY WIEDEREINFÜGUNG VON LITHIUM IN VERWERTETE ELEKTRODENMATERIALIEN FÜR BATTERIEN RÉINTRODUCTION DE LITHIUM DANS DES MATÉRIAUX D’ÉLECTRODES RECYCLÉS POUR BATTERIES (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-A1- 1 009 058 CN-A- 1 585 187 HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL JP-A- 11 054 159 US-A- 5 628 973 PT RO SE SI SK TR US-A- 5 679 477 US-A- 6 150 050 US-A1- 2003 180 604 US-A1- 2004 175 618 (30) Priority: 22.02.2008 US 30916 US-A1- 2005 003 276 US-A1- 2005 175 877 US-A1- 2005 244 704 US-A1- 2007 134 546 (43) Date of publication of application: US-A1- 2007 224 508 US-B1- 6 261 712 10.11.2010 Bulletin 2010/45 US-B2- 6 844 103 (73) Proprietor: Sloop, Steven E. -

P880-183197 Ii I 11\11111 Ii Iii Ii 11111 1111111 1111111
EPA-560/ll-80-005 P880-183197 II I 11\11111 II III II 11111 1111111 1111111 INVESTIGATION OF SELECTED POTENTIAL ENVIRONMENTAL CONTAMINANTS: EPOXIDES Dennis A. Bogyo Sheldon S. Lande William M. Meylan Philip H. Howard Joseph Santodonato March 1980 FINAL REPORT Contract No. 68-01-3920 SRC No. L1342-05 Project Officer - Frank J. Letkiewicz Prepared for: Office of Toxic Substances U.S. Environmental Protection Agency Washington, D.C. 20460 Document is available to the public through the National Technical Information Service, Springfield, Virginia 22151 REPRODUCED BY CE U S DEPARTMENT OF COMMER ., NATIONAL TECHNICAL INFORMATION SERVICE SPRINGFIELD, VA 22161 TECHNICAL REPORT DATA (Please read illWtictiollS on the reverse before completing) I REPORT NO. 2 3. RECIPIENT'S ACCESSIO~NO. EPA-560/ll-80-005 1 . ~~~~ llf03:;n~? -l. TITLE AND SUBTITLE S. REPORT DATE Investigation of Selected Potential March 1980 Environmental Contaminants: Epoxides 6. PERFORMING ORGANIZATION CODE 7 AuTHORiS) 8. PERFORMING ORGANIZATION REPORT NO Dennis A. Bogyo, Sheldon S. Lande, William M. Meylan, TR 80;...535 Philip H. Howard, Joseph Santodonato 9. PERFORMING ORGANIZATION NAME AND ADDRESS 10. PROGRAM ELEMENT NO. Center for Chemical Hazard Assessment Syracuse Research Corporation 11. CONTRACT/GRANT NO. Merrill Lane EPA 68-01-3920 Syracuse, New York 13210 12. SPONSORING AGENCY NAME AND ADDRESS 13. TYPE OF REPORT AND PERIOD COVERED Final Technical Report - Office of· Toxic Substances 14. SPONSORING AGENCY CODE U.S. Environmental Protection Agency Washington, D.C. 20460 15. SUPPLEMENTARY NOTES 16. ABSTRACT This report reviews the potential environmental and health hazards associated with the commercial use of selected epoxide compounds. -

Alkylation of Enolates Clayden.Pdf
Alkylation of enolates 26 Connections Building on: Arriving at: Looking forward to: • Enols and enolates ch21 • How to make new C–C bonds using • Forming C–C bonds by reacting • Electrophilic addition to alkenes ch20 carbonyl compounds as nucleophiles nucleophilic enolates with electrophilic carbonyl compounds Nucleophilic substitution reactions • How to prevent carbonyl compounds • ch27 ch17 reacting with themselves • Forming C–C bonds by reacting nucleophilic enolates with electrophilic carboxylic acid derivatives ch28 • Forming C–C bonds by reacting nucleophilic enolates with electrophilic alkenes ch29 • Retrosynthetic analysis ch30 Chapters 26–29 continue the theme of synthesis that started with Chapter 24 and will end with Chapter 30. This group of four chapters introduces the main C–C bond-forming reactions of enols and enolates. We develop the chemistry of Chapter 21 with a discussion of enols and enolates attack- ing to alkylating agents (Chapter 26), aldehydes and ketones (Chapter 27), acylating agents (Chapter 28), and electrophilic alkenes (Chapter 29). Carbonyl groups show diverse reactivity In earlier chapters we discussed the two types of reactivity displayed by the carbonyl group. We first described reactions that involve nucleophilic attack on the carbon of the carbonyl, and in Chapter 9 we showed you that these are among the best ways of making new C–C bonds. In this chapter we shall again be making new C–C bonds, but using electrophilic attack on carbonyl compounds: in other words, the carbonyl compound carbonyl compound acts as an electrophile will be reacting as the nucleophile in R R R the reaction. We introduced the Nu nucleophilic forms of carbonyl com- O O OH pounds—enols, and enolates—in Nu Nu Chapter 21. -

Synthesis of (+)-Lineariifolianone and Related Cyclopropenone- Containing Sesquiterpenoids Keith P
Article Cite This: J. Org. Chem. 2019, 84, 5524−5534 pubs.acs.org/joc Synthesis of (+)-Lineariifolianone and Related Cyclopropenone- Containing Sesquiterpenoids Keith P. Reber,*,† Ian W. Gilbert,† Daniel A. Strassfeld,‡ and Erik J. Sorensen§ † Department of Chemistry, Towson University, Towson, Maryland 21252, United States ‡ Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States § Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: A synthesis of the proposed structure of lineariifolianone has been achieved in eight steps and 9% overall yield starting from (+)-valencene, leading to a reassignment of the absolute configuration of this unusual cyclopropenone-containing natural product. Key steps in the synthetic route include kinetic protonation of an enolate to epimerize the C7 stereocenter and a stereoconvergent epoxide opening to establish the trans-diaxial diol functionality. The syntheses of the enantiomers of two other closely related natural products are also reported, confirming that all three compounds belong to the eremophilane class of sesquiterpenoids. ■ INTRODUCTION derivatives,10 and the parent compound has even been 11 Plants of the genus Inula have been widely used in traditional detected in interstellar space. Chinese medicine for their anti-inflammatory properties and to Natural products containing the cyclopropenone group are 1 quite rare, and to date only six other examples have been treat bacterial infections. In 2010, Zhang and Jin reported the 12 isolation of the sesquiterpenoid lineariifolianone (1), which isolated (Figure 2). Penitricin (2) and alutacenoic acids A was obtained from the aerial parts of the flowering plant Inula lineariifolia (Figure 1).2 The structure of this natural product Downloaded via TOWSON UNIV on July 13, 2020 at 20:20:52 (UTC).