Acute Disseminated Encephal Omyelitis (ADEM) After Pneumococcal Meningitis in a Child
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Myalgic Encephalomyelitis/Chronic Fatigue
2019 Science & Discovery Webinar Series ME/CFS in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity with Amy Proal, Ph.D. November 14, 2019 | 1:00 PM Eastern www.SolveME.org About Our Webinars • Welcome to the 2019 Webinar Series! • The audience is muted; use the question box to send us questions. Dr. Proal will address as many questions as time permits at the end of the webinar • Webinars are recorded and the recording is made available on our YouTube channel http://youtube.com/SolveCFS • The Solve ME/CFS Initiative does not provide medical advice www.SolveCFS.org 2019 Science & Discovery Webinar Series ME/CFS in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity with Amy Proal, Ph.D. November 14, 2019 | 1:00 PM Eastern www.SolveME.org Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity Amy Proal, Autoimmunity Research Foundation/PolyBio Millions of patients across the globe are suffering with myalgic encephalomyelitis (ME/CFS) Currently there is no one disease-specific biomarker and severely ill patients are often wheelchair dependent, bedridden and unable to perform basic tasks of work or daily living. #millionsmissing Myalgic Encephalomeylitis (ME) = swelling of the brain • Unrelenting fatigue that does -

African Meningitis Belt
WHO/EMC/BAC/98.3 Control of epidemic meningococcal disease. WHO practical guidelines. 2nd edition World Health Organization Emerging and other Communicable Diseases, Surveillance and Control This document has been downloaded from the WHO/EMC Web site. The original cover pages and lists of participants are not included. See http://www.who.int/emc for more information. © World Health Organization This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes. The views expressed in documents by named authors are solely the responsibility of those authors. The mention of specific companies or specific manufacturers' products does no imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. CONTENTS CONTENTS ................................................................................... i PREFACE ..................................................................................... vii INTRODUCTION ......................................................................... 1 1. MAGNITUDE OF THE PROBLEM ........................................................3 1.1 REVIEW OF EPIDEMICS SINCE THE 1970S .......................................................................................... 3 Geographical distribution -
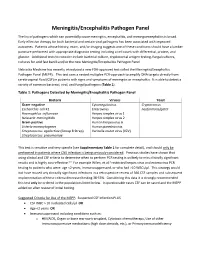
Meningitis/Encephalitis Pathogen Panel
Meningitis/Encephalitis Pathogen Panel The list of pathogens which can potentially cause meningitis, encephalitis, and meningoencephalitis is broad. Early effective therapy for both bacterial and certain viral pathogens has been associated with improved outcomes. Patients whose history, exam, and/or imaging suggests one of these conditions should have a lumber puncture performed with appropriate diagnostic testing including a cell count with differential, protein, and glucose. Additional tests to consider include bacterial culture, cryptococcal antigen testing, fungal cultures, cultures for acid fast bacilli and/or the new Meningitis/Encephalitis Pathogen Panel. Nebraska Medicine has recently introduced a new FDA-approved test called the Meningitis/Encephalitis Pathogen Panel (MEPP). This test uses a nested multiplex PCR-approach to amplify DNA targets directly from cerebrospinal fluid (CSF) in patients with signs and symptoms of meningitis or encephalitis. It is able to detect a variety of common bacterial, viral, and fungal pathogens (Table 1). Table 1: Pathogens Detected by Meningitis/Encephalitis Pathogen Panel Bacteria Viruses Yeast Gram-negative Cytomegalovirus Cryptococcus Escherichia coli K1 Enterovirus neoformans/gattii Haemophilus influenzae Herpes simplex virus 1 Neisseria meningitidis Herpes simplex virus 2 Gram-positive Human herpesvirus 6 Listeria monocytogenes Human parechovirus Streptococcus agalactiae (Group B Strep) Varicella zoster virus (VZV) Streptococcus pneumoniae This test is sensitive and very specific (see Supplementary Table 1 for complete detail), and should only be performed in patients where CNS infection is being seriously considered. Previous studies have shown that using clinical and CSF criteria to determine when to perform PCR testing is unlikely to miss clinically significant results and is highly cost-effective.1-3 For example Wilen, et al.3 restricted herpes virus and enterovirus PCR testing to patients who were: age <2 years, immunosuppressed, or who had >10 WBCs/µl. -

Bacterial Meningitis and Neurological Complications in Adults
OCUSED EVIEW Parunyou J. et al. Bacterial meningitis F R Bacterial meningitis and neurological complications in adults Parunyou Julayanont MD, Doungporn Ruthirago MD, John C. DeToledo MD ABSTRACT Bacterial meningitis is a leading cause of death from infectious disease worldwide. The neurological complications secondary to bacterial meningitis contribute to the high mortality rate and to disability among the survivors. Cerebrovascular complications, including infarc- tion and hemorrhage, are common. Inflammation and increased pressure in the subarach- noid space result in cranial neuropathy. Seizures occur in either the acute or delayed phase after the infection and require early detection and treatment. Spreading of infection to other intracranial structures, including the subdural space, brain parenchyma, and ventricles, in- creases morbidity and mortality in survivors. Infection can also spread to the spinal canal causing spinal cord abscess, epidural abscess, polyradiculitis, and spinal cord infarction secondary to vasculitis of the spinal artery. Hypothalamic-pituitary dysfunction is also an un- common complication after bacterial meningitis. Damage to cerebral structures contributes to cognitive and neuropsychiatric problems. Being aware of these complications leads to early detection and treatment and improves mortality and outcomes in patients with bacte- rial meningitis. Key words: meningitis; meningitis, bacterial; central nervous system bacterial infection; nervous system diseases INTRODUCTION prove recovery and outcomes. Bacterial meningitis is a leading cause of death In this article, we present a case of bacterial men- from infectious disease worldwide. Despite the avail- ingitis complicated by an unusual number of neuro- ability of increasingly effective antibiotics and inten- logical complications that occurred in spite of a timely sive neurological care, the overall mortality remains diagnosis, adequate treatment, and intensive neuro- high, with 17-34% of the survivors having unfavorable logical monitoring. -

Chapter 18: Polio
Poliomyelitis Concepcion F. Estivariz, MD; Ruth Link-Gelles, PhD, MPH; and Tom Shimabukuro, MD, MPH, MBA Descriptions of polio-like illnesses have been around since antiquity, including a funerary stele depicting a man with Poliomyelitis a withered leg leaning on a staff. Michael Underwood first ● First described by Michael described a debility of the lower extremities in children that was Underwood in 1789 recognizable as poliomyelitis in England in 1789, but the disease ● Developed countries in was not observed in epidemics until the late 19th century. Northern Hemisphere During the first half of the 20th century, developed countries in suffered increasingly severe the Northern Hemisphere suffered epidemics each summer and epidemics in the first half fall that became increasingly severe. Polio infections peaked in of the 20th century the United States in 1952, with more than 21,000 paralytic cases. Following introduction of effective vaccines in 1955 (inactivated ● More than 21,000 paralytic polio vaccine, IPV) and 1961 (oral poliovirus vaccine, OPV), cases reported in the U.S. polio incidence declined rapidly. The last case of wild poliovirus in 1952 acquired in the United States was in 1979. ● Last case of wild poliovirus acquired in the U.S. was 1979 Poliovirus Poliovirus is a member of the enterovirus subgroup, family Picornaviridae. Picornaviruses are small, ether-insensitive viruses Poliovirus with an RNA genome. ● Enterovirus (RNA) ● Three serotypes: type 1, type 2, There are three poliovirus serotypes (type1, type 2, and type type 3 3); immunity to one serotype does not produce significant immunity to the other serotypes. ● Immunity to one serotype does not produce significant Poliovirus is rapidly inactivated by heat, formaldehyde, chlorine, immunity to other serotypes and ultraviolet light. -

Chronic Fatigue Syndrome
Ministry of Defence Synopsis of Causation Chronic Fatigue Syndrome Author: Dr Adrian Roberts, Medical Author, Medical Text, Edinburgh Validator: Dr Selwyn Richards, Poole Hospital NHS Trust, Poole, Dorset September 2008 Disclaimer This synopsis has been completed by medical practitioners. It is based on a literature search at the standard of a textbook of medicine and generalist review articles. It is not intended to be a meta- analysis of the literature on the condition specified. Every effort has been taken to ensure that the information contained in the synopsis is accurate and consistent with current knowledge and practice and to do this the synopsis has been subject to an external validation process by consultants in a relevant specialty nominated by the Royal Society of Medicine. The Ministry of Defence accepts full responsibility for the contents of this synopsis, and for any claims for loss, damage or injury arising from the use of this synopsis by the Ministry of Defence. 2 1. Definition 1.1 Chronic fatigue syndrome (CFS) is a significant illness that causes severe disabling physical and mental fatigue exacerbated by minimal exertion, in the absence of any conventional physical or psychological disorder to explain the problem. The term “chronic fatigue syndrome” was conceived relatively recently. However, the symptom complex that it describes has been recognised for over a century, during which time it has been classified under a variety of titles including neurasthenia, Royal Free disease, myalgic encephalomyelitis (ME), and post-viral fatigue syndrome. 1.2 CFS is defined by symptoms and disability and has no confirmatory physical signs or characteristic laboratory abnormalities. -

Viral Meningitis/Encephalitis Public Health Communicable Disease Control Unit
Communicable Disease Management Protocol Manitoba Health Viral Meningitis/Encephalitis Public Health Communicable Disease Control Unit Case Definition Etiology Clinically compatible illness and laboratory- Numerous viruses can cause this syndrome, but half confirmed virus identification using serologic or or more of cases have no demonstrable etiology. In isolation techniques. Canada, enteroviruses cause most cases of known etiology, particularly coxsackievirus and echovirus. Reporting Requirements In addition, arboviruses, measles, herpes simplex and varicella viruses, adenovirus and others are • Positive isolates or positive serologic tests for viral responsible for sporadic cases. meningitis/encephalitis are reportable by laboratory. Epidemiology • Clinical cases of viral meningitis/encephalitis Reservoir: Humans and probably certain birds, need not be reported by attending health care mammals and reptiles. professional, unless the case results in neurological sequelae or death. Transmission: Depends on specific virus, but for enteroviruses, generally directly by fecal-oral or • Meningitis/encephalitis due to Western Equine respiratory droplet contact with an infected person, Encephalitis, measles, mumps or rubella should or indirectly by contact with articles freshly soiled be reported under those diseases. with feces or throat discharges from an infected person. Western equine encephalitis is transferred Clinical Presentation/Natural History through bites by infected mosquitoes. Viral meningitis/encephalitis is a relatively common Occurrence: but rarely serious syndrome with multiple viral General: Some viruses have a worldwide etiologies. It usually appears as a sudden onset of distribution, others are localized. Cases may be fever, with headache, and other signs and sporadic or occur in epidemics. Seasonal symptoms of meningeal involvement and abnormal increases in late summer and early autumn are CSF findings. -

7. Headache Attributed to Non-Vascular Intracranial Disorder
Classification and Diagnosis of Secondary Headaches, Part II- Altered Intracerebral Pressure, Neoplasms, and Infections Lawrence C. Newman, M.D. Director, The Headache Institute Roosevelt Hospital Center New York, N.Y. 7. Headache7. Headache attributed attributed to to non-vascularnon-vascular intracranial intracranial disorder 7.1 Headache attributed to high cerebrospinal fluid pressure 7.2 Headache attributed to low cerebrospinaldisorder fluid pressure 7.3 Headache attributed to non-infectious inflammatory disease 7.4 Headache attributed to intracranial neoplasm 7.5 Headache attributed to intrathecal injection 7.6 Headache attributed to epileptic seizure 7.7 Headache attributed to Chiari malformation type I 7.8 Syndrome of transient Headache and Neurological Deficits with cerebrospinal fluid Lymphocytosis (HaNDL) 7.9 Headache attributed to other non-vascular intracranial disorder ICHD-II. Cephalalgia 2004; 24 (Suppl 1) ©International Headache Society 2003/4 Headaches attributed to alterations in CSF pressure: • Headache frequently accompanies alteration of CSF pressure, either high or low • Pressure alterations may be the result of disruptions of CSF production, flow, or absorption • Major source of CSF is choroid plexus; some also formed extra-choroidal • CSF absorbed primarily in pacchionian granulations arachnoid villi and vessels of subarachnoid space over hemispheres ® American Headache Society Increased Intracranial Pressure: Secondary Causes • Venous sinus occlusion • Medications (naladixic • Radical neck dissection acid,danocrine, -

Enteroviral Meningitis Reduces CSF Concentration of Aβ42, but Does Not Affect Markers of Parenchymal Damage
European Journal of Clinical Microbiology & Infectious Diseases (2019) 38:1443–1447 https://doi.org/10.1007/s10096-019-03569-0 ORIGINAL ARTICLE Enteroviral meningitis reduces CSF concentration of Aβ42, but does not affect markers of parenchymal damage Kacper Toczylowski1 & Malgorzata Wojtkowska2 & Artur Sulik1 Received: 19 March 2019 /Accepted: 23 April 2019 /Published online: 15 May 2019 # The Author(s) 2019 Abstract Biomarkers classically studied in Alzheimer’s disease have been analyzed in numerous central nervous system infections in adults, but there are scarce data on these biomarkers in children. Enteroviruses appear to be the most common cause of aseptic meningitis throughout the world. The aim of the study was to investigate neuroinflammatory properties of non-polio enterovi- ruses by measuring CSF concentrations of biomarkers that are involved in neuropathological pathways of neurodegenerative disorders. We measured Aβ42, t-tau, and S100B concentrations in 42 children with enteroviral meningitis (EM) compared to control group without central nervous system infection. We found enteroviral meningitis (EM) to reduce CSF concentration of Aβ42 (median, 1051.1 pg/mL; interquartile range (IQR), 737.6–1559.5 vs. median, 459.4 pg/mL; IQR, 312.0–662.0, p <0.001). In contrast, CSF concentrations of t-tau and S100B were not affected by EM. There was a correlation between total neutrophil count in CSF and Aβ42 (R = − 0.59, p < 0.001). Absolute number of mononuclear cells in the CSF correlated with CSF t-tau (R =0.41,p < 0.05). Both correlations remained significant after adjustment for age, blood leukocytes, serum CRP, CSF leuko- cytes, and CSF protein concentration. -

Viral Meningitis- FAQ (Aseptic Meningitis, Non-Bacterial Meningitis)
Viral Meningitis- FAQ (aseptic meningitis, non-bacterial meningitis) What is viral meningitis? Viral meningitis is the most common type of meningitis, an inflammation of the tissue that covers the brain and spinal cord. It is often less severe than bacterial meningitis, and most people get better on their own (without treatment). However, it’s very important for anyone with symptoms of meningitis to see a healthcare provider as soon as possible because some types of meningitis can be very serious, and only a doctor can determine the type of disease you have. What causes viral meningitis? Non-polio enteroviruses are the most common cause of viral meningitis in the United States, especially from late summer to early fall when these viruses spread most often. However, only a small number of people who get infected with enteroviruses will actually develop meningitis. Other viruses that can cause meningitis are mumps virus, measles virus, herpes viruses, influenza virus, and arboviruses. Who can get viral meningitis? Anyone can get viral meningitis, but the illness is more often seen in children and people with weakened immune systems. How do you get viral meningitis? Viral meningitis is usually caused by enteroviruses, viruses commonly found in respiratory droplets (sneezes, coughs, spit) and stool. The virus can then pass from one person to another through close contact, such as touching or shaking hands, with an infected person or by touching objects or surfaces that have the virus on them, then touching your eyes, nose, or mouth before washing your hands. Also, changing diapers of an infected person, then touching your eyes, nose, or mouth before washing your hands could expose you to the virus. -

Meningitis - Hospital Admission
Meningitis - Hospital Admission Meningitis (men-in-JIE-tiss) is an infection of the meninges (men-IN-jeez). These are the membranes that cover the brain and spinal cord (Picture 1). This disease is more common in infants and young children than in adults. Children with cochlear implants are at increased risk for meningitis. Vertebrae (bones of the spine) Meningitis is caused by germs - either viruses (viral meningitis) or bacteria (bacterial meningitis). The germs that cause the illness usually come from the nose and throat. Then they spread through L.P. site the bloodstream to the meninges. Meninges Spinal cord Bacterial meningitis is much more serious than Picture 1 The meninges cover viral meningitis. The side effects may be more severe. the brain and spinal cord. Symptoms appear after a cold or sore throat or there may be no other illness just before symptoms come on. If meningitis is suspected, the child or adult should be seen by a doctor right away. Bacterial meningitis occurs most often in the winter. It is caused by several different types of bacteria (see chart). Hearing loss is the most common complication. Viral meningitis occurs most often in the summer. It usually causes a fairly mild illness. Complications can develop but they are rare. There is no vaccine against most causes of viral meningitis. A vaccine against the bacteria Streptococcus pneumonae is recommended by the Academy of Pediatrics for children including those receiving cochlear implants. Vaccine against Haemophilus influenzae meningitis is very effective. Vaccine against Neisseria meningitis is recommended for some children. Signs and Symptoms Bacterial Meningitis Viral Meningitis Cause Streptococcus pneumoniae Several different viruses Neisseria meningitidis Haemophilus influenzae Continued on page 2 HH-I-89 9/78, Revised 11/10 Copyright 1978-2010, Nationwide Children's Hospital Meningitis Page 2 of 4 Signs and Symptoms, continued Bacterial Meningitis Viral Meningitis Early Signs . -

Polio: Questions and Answers Q&A Information About the Disease and Vaccines
Polio: Questions and Answers Q&A information about the disease and vaccines What causes polio? adulthood (usually after an interval of 30–40 years). Polio is caused by a virus. This problem is called post-polio syndrome (PPS) and symptoms can include new muscle pain, weak- How does polio spread? ness, or paralysis. PPS is not infectious. For more information or for support for people with post-polio Polio is usually spread via the fecal-oral route (i.e., syndrome, go to www.post-polio.org. the virus is transmitted from the stool of an infected person to the mouth of another person from con- How is polio diagnosed? taminated hands or such objects as eating utensils). Some cases may be spread directly via an oral to If a person is suspected of being infected, a sample oral route. from their stool or throat should be tested for the poliomyelitis virus. How long does it take to show signs of polio after being exposed? How long is a person with polio contagious? Patients infected with the polio virus can pass the The incubation period for polio is commonly 6–20 virus on for 7–10 days before the onset of disease. In days, with a range of 3–35 days. addition, they can continue to shed the virus in their What are the symptoms of polio? stool for 3–6 weeks. Surprisingly, 95% of all individuals infected with polio Is there a treatment for polio? have no apparent symptoms. There is no “cure” for polio. People infected with Another 4%–8% of infected individuals have symp- polio need supportive therapy, such as bed rest and toms of a minor, non-specific nature, such as sore fluids.