Synthesis, in Vitro Urease Inhibitory Activity, and Molecular Docking Studies of (Perfluorophenyl)Hydrazone Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Institute of Business Administration, Karachi Bba, Bs
FINAL RESULT - FALL 2020 ROUND 1 Announced on Tuesday, February 25, 2020 INSTITUTE OF BUSINESS ADMINISTRATION, KARACHI BBA, BS (ACCOUNTING & FINANCE), BS (ECONOMICS) & BS (SOCIAL SCIENCES) ADMISSIONS TEST HELD ON SUNDAY, FEBRUARY 9, 2020 (FALL 2020, ROUND 1) LIST OF SUCCESSFUL CANDIDATES FOR DIRECT ADMISSION (BSAF PROGRAM) SAT Test Math Eng TOTAL Maximum Marks 800 800 1600 Cut-Off Marks 600 600 1410 Math Eng Total IBA Test MCQ MCQ MCQ Maximum Marks 180 180 360 Cut-Off Marks 100 100 256 Seat S. No. App No. Name Father's Name No. 1 18 845 FABIHA SHAHID SHAHIDSIDDIQUI 132 136 268 2 549 1510 MUHAMMAD QASIM MAHMOOD AKHTAR 148 132 280 3 558 426 MUHAMMAD MUTAHIR ABBAS AMAR ABBAS 144 128 272 4 563 2182 ALI ABDULLAH MUHAMMAD ASLAM 136 128 264 5 1272 757 MUHAMMAD DANISH NADEEM MUHAMMAD NADEEM TAHIR 136 128 264 6 2001 1 MUHAMMAD JAWWAD HABIB MUHAMMAD NADIR HABIB 160 100 260 7 2047 118 MUHAMMAD ANAS LIAQUAT ALI 148 128 276 8 2050 125 HAFSA AZIZ AZIZAHMED 128 136 264 9 2056 139 MUHAMMAD SALMAN ANWAR MUHAMMAD ANWAR 156 132 288 10 2086 224 MOHAMMAD BADRUDDIN RIND BALOCH BAHAUDDIN BALOCH 144 112 256 11 2089 227 AHSAN KAMAL LAGHARI GHULAM ALI LAGHARI 136 160 296 12 2098 247 SYED MUHAMMAD RAED SYED MUJTABA NADEEM 152 132 284 13 2121 304 AREEB AHMED BAIG ILHAQAHMED BAIG 108 152 260 14 2150 379 SHAHERBANO ‐ ABDULSAMAD SURAHIO 148 124 272 15 2158 389 AIMEN ATIQ SYED ATIQ UR REHMAN 148 124 272 16 2194 463 MUHAMMAD SAAD MUHAMMAD ABBAS 152 136 288 17 2203 481 FARAZ NAWAZ MUHAMMAD NAWAZ 132 128 260 18 2210 495 HASNAIN IRFAN IRFAN ABDULAZIZ 128 132 260 19 2230 -

List of Category -I Members Registered in Membership Drive-Ii
LIST OF CATEGORY -I MEMBERS REGISTERED IN MEMBERSHIP DRIVE-II MEMBERSHIP CGN QUOTA CATEGORY NAME DOB BPS CNIC DESIGNATION PARENT OFFICE DATE MR. DAUD AHMAD OIL AND GAS DEVELOPMENT COMPANY 36772 AUTONOMOUS I 25-May-15 BUTT 01-Apr-56 20 3520279770503 MANAGER LIMITD MR. MUHAMMAD 38295 AUTONOMOUS I 26-Feb-16 SAGHIR 01-Apr-56 20 6110156993503 MANAGER SOP OIL AND GAS DEVELOPMENT CO LTD MR. MALIK 30647 AUTONOMOUS I 22-Jan-16 MUHAMMAD RAEES 01-Apr-57 20 3740518930267 DEPUTY CHIEF MANAGER DESTO DY CHEIF ENGINEER CO- PAKISTAN ATOMIC ENERGY 7543 AUTONOMOUS I 17-Apr-15 MR. SHAUKAT ALI 01-Apr-57 20 6110119081647 ORDINATOR COMMISSION 37349 AUTONOMOUS I 29-Jan-16 MR. ZAFAR IQBAL 01-Apr-58 20 3520222355873 ADD DIREC GENERAL WAPDA MR. MUHAMMA JAVED PAKISTAN BORDCASTING CORPORATION 88713 AUTONOMOUS I 14-Apr-17 KHAN JADOON 01-Apr-59 20 611011917875 CONTRALLER NCAC ISLAMABAD MR. SAIF UR REHMAN 3032 AUTONOMOUS I 07-Jul-15 KHAN 01-Apr-59 20 6110170172167 DIRECTOR GENRAL OVERS PAKISTAN FOUNDATION MR. MUHAMMAD 83637 AUTONOMOUS I 13-May-16 MASOOD UL HASAN 01-Apr-59 20 6110163877113 CHIEF SCIENTIST PROFESSOR PAKISTAN ATOMIC ENERGY COMMISION 60681 AUTONOMOUS I 08-Jun-15 MR. LIAQAT ALI DOLLA 01-Apr-59 20 3520225951143 ADDITIONAL REGISTRAR SECURITY EXCHENGE COMMISSION MR. MUHAMMAD CHIEF ENGINEER / PAKISTAN ATOMIC ENERGY 41706 AUTONOMOUS I 01-Feb-16 LATIF 01-Apr-59 21 6110120193443 DERECTOR TRAINING COMMISSION MR. MUHAMMAD 43584 AUTONOMOUS I 16-Jun-15 JAVED 01-Apr-59 20 3820112585605 DEPUTY CHIEF ENGINEER PAEC WASO MR. SAGHIR UL 36453 AUTONOMOUS I 23-May-15 HASSAN KHAN 01-Apr-59 21 3520227479165 SENOR GENERAL MANAGER M/O PETROLEUM ISLAMABAD MR. -

Pld 2017 Sc 70)
IN THE SUPREME COURT OF PAKISTAN (Original Jurisdiction) PRESENT: Mr. Justice Asif Saeed Khan Khosa Mr. Justice Ejaz Afzal Khan Mr. Justice Gulzar Ahmed Mr. Justice Sh. Azmat Saeed Mr. Justice Ijaz ul Ahsan Constitution Petition No. 29 of 2016 (Panama Papers Scandal) Imran Ahmad Khan Niazi Petitioner versus Mian Muhammad Nawaz Sharif, Prime Minister of Pakistan / Member National Assembly, Prime Minister’s House, Islamabad and nine others Respondents For the petitioner: Syed Naeem Bokhari, ASC Mr. Sikandar Bashir Mohmad, ASC Mr. Fawad Hussain Ch., ASC Mr. Faisal Fareed Hussain, ASC Ch. Akhtar Ali, AOR with the petitioner in person Assisted by: Mr. Yousaf Anjum, Advocate Mr. Kashif Siddiqui, Advocate Mr. Imad Khan, Advocate Mr. Akbar Hussain, Advocate Barrister Maleeka Bokhari, Advocate Ms. Iman Shahid, Advocate, For respondent No. 1: Mr. Makhdoom Ali Khan, Sr. ASC Mr. Khurram M. Hashmi, ASC Mr. Feisal Naqvi, ASC Assisted by: Mr. Saad Hashmi, Advocate Mr. Sarmad Hani, Advocate Mr. Mustafa Mirza, Advocate For the National Mr. Qamar Zaman Chaudhry, Accountability Bureau Chairman, National Accountability (respondent No. 2): Bureau in person Mr. Waqas Qadeer Dar, Prosecutor- Constitution Petition No. 29 of 2016, 2 Constitution Petition No. 30 of 2016 & Constitution Petition No. 03 of 2017 General Accountability Mr. Arshad Qayyum, Special Prosecutor Accountability Syed Ali Imran, Special Prosecutor Accountability Mr. Farid-ul-Hasan Ch., Special Prosecutor Accountability For the Federation of Mr. Ashtar Ausaf Ali, Attorney-General Pakistan for Pakistan (respondents No. 3 & Mr. Nayyar Abbas Rizvi, Additional 4): Attorney-General for Pakistan Mr. Gulfam Hameed, Deputy Solicitor, Ministry of Law & Justice Assisted by: Barrister Asad Rahim Khan Mr. -
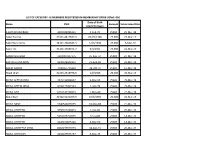
Name CNIC Date of Birth (Dd/Mm/Yyyy) Amount Submission
LIST OF CATEGORY -III MEMBERS REGISTERED IN MEMBERSHIP DRIVE-II(Part-4th) Date of Birth Name CNIC Amount Submission Date (dd/mm/yyyy) A SATTAR UMERANI 4200036095311 1-Feb-70 25000 25-Mar-19 Aaber Farooq 33301-4817637-3 03/09/1986 25,000 25-Mar-19 Aabis Raza Kazmi 34101-7982643-3 14/9/1990 25,000 6-Mar-19 Aamir Ali 31101-3366151-7 9/9/1991 25,000 21-Mar-19 AAMIR SHAHZAD 3310007107175 25-Sep-72 25000 21-Mar-19 AASHIQ ALI MEMON 4330329942951 21-Aug-69 25000 22-Mar-19 AASIM BASHIR 3740567745419 20-Oct-70 25000 11-Mar-19 Abaid Ullah 32103-2518976-5 1/6/1966 25,000 25-Mar-19 ABDUL ALEEM KHAN 3520230658047 9-Mar-65 25000 25-Mar-19 ABDUL AZEEM JATOI 4230177451543 1-Apr-78 25000 25-Mar-19 ABDUL AZIZ 6110142134913 1-May-64 25000 22-Mar-19 Abdul Bari 32402-5221299-9 10/9/1965 25,000 25-Mar-19 ABDUL BASIT 3740540476955 24-Mar-88 25000 21-Mar-19 ABDUL GHAFFAR 3550102289413 1-Apr-89 25000 25-Mar-19 ABDUL GHAFFAR 5210105714535 17-Jul-80 25000 14-Mar-19 ABDUL GHAFFAR 4220102855149 4-Mar-60 25000 14-Mar-19 ABDUL GHAFFFAR KHAN 8210299699793 18-Dec-71 25000 25-Mar-19 ABDUL GHAFOOR 4230109573757 6-Mar-75 25000 25-Mar-19 Date of Birth Name CNIC Amount Submission Date (dd/mm/yyyy) ABDUL GHAFOOR 3740615687037 22-Oct-63 25000 22-Mar-19 ABDUL GHAFOOR SANJRANI 4230103641849 30-May-57 25000 18-Mar-19 ABDUL HAFEEZ 4130381796287 2-Mar-85 25000 18-Mar-19 Abdul Hameed 35202-5798517-7 13/11/1951 25,000 13-Mar-19 ABDUL HAMEED 4240116994783 20-Sep-62 25000 25-Mar-19 ABDUL HAMID 4210178803863 12-Mar-63 25000 22-Mar-19 ABDUL HAMID KHAN 4210115977797 12-Jan-64 25000 25-Mar-19 -

Project of Support to the National Time Bound Programme on The
Project of Support to the National Time Bound Program on the Worst Forms of Child Labor in Pakistan (2003-2008) ILO Project No: PAK/03/P50/USA Final Expanded Evaluation Report Evaluation Team Sarah Tirmazi, Ph.D., International Consultant & Team Leader Syed Mohammad Ali, National Consultant & Impact Assessment Team of the Sustainable Development Policy Institute (SDPI) Dr. Abid Suleri (Executive Director, SDPI), Kiran Habib and Syed Asghar Shah October 28, 2008 Islamabad (This report has not been professionally edited) 1 ACRONYMS APSO Action Program Summary Outline BLCC Basic Learning Community Centers (NGO) BLS Baseline Survey Bunyad NGO, IP, POSTBP, Sialkot CCB Citizen’s Community Board CIWCE Center for Improvement of Working Conditions and Environment, GoPunjab CL Child labor CLMS Child Labour Monitoring System CRC Convention on the Rights of the Child CTA Chief Technical Adviser DCC District Coordination Committee DCO District Coordination Officer DED Design, Evaluation and Documentation Section, ILO, Geneva DEP District Education Plan DG District Government DPNet Development Policy Network ECA Employment of Children Act, 1991 EDO Executive District Officer EDO-CD Executive District Officer for Community Development EFA Education for All EFP Employers Federation of Pakistan EMIS Education Management Information System ESR Education Sector Reforms FBS Federal Bureau of Statistics GoP Government of Pakistan IAS Impact Assessment Survey for Rag-pickers, Rawalpindi ILO International Labor Office IP Implementing partner IPEC International -

Supplemental Statement Washington, DC 20530 Pursuant to the Foreign Agents Registration Act of 1938, As Amended
Received by NSD/FARA Registration Unit 07/17/2013 12:53:25 PM OMB NO. 1124-0002; Expires February 28, 2014 «JJ.S. Department of Justice Supplemental Statement Washington, DC 20530 Pursuant to the Foreign Agents Registration Act of 1938, as amended For Six Month Period Ending 06/30/2013 (Insert date) I - REGISTRANT 1. (a) Name of Registrant (b) Registration No. Pakistan Tehreek e Insaf 5975 (c) Business Address(es) of Registrant 315 Maple street Richardson TX, 75081 Has there been a change in the information previously furnished in connection with the following? (a) If an individual: (1) Residence address(es) Yes Q No D (2) Citizenship Yes Q No Q (3) Occupation Yes • No D (b) If an organization: (1) Name Yes Q No H (2) Ownership or control Yes • No |x] - (3) Branch offices Yes D No 0 (c) Explain fully all changes, if any, indicated in Items (a) and (b) above. IF THE REGISTRANT IS AN INDIVIDUAL, OMIT RESPONSE TO ITEMS 3,4, AND 5(a). 3. If you have previously filed Exhibit C1, state whether any changes therein have occurred during this 6 month reporting period. Yes D No H If yes, have you filed an amendment to the Exhibit C? Yes • No D If no, please attach the required amendment. I The Exhibit C, for which no printed form is provided, consists of a true copy of the charter, articles of incorporation, association, and by laws of a registrant that is an organization. (A waiver of the requirement to file an Exhibit C may be obtained for good cause upon written application to the Assistant Attorney General, National Security Division, U.S. -

Hackney Carriage/Private Hire Drivers
STOCKTON-ON-TEES BOROUGH COUNCIL - REGISTER OF LICENSED HACKNEY CARRIAGE/PRIVATE HIRE DRIVERS THIS REGISTER WILL BE UPDATED EVERY 12 WEEKS (LAST UPDATE: 14/06/21) TYPE OF LICENCE LICENCE NAME START DATE EXPIRY DATE NUMBER Private Hire Driver DRV 002 Mr Kashaf Moghal 01/05/2021 30/04/2022 Hackney Carriage Driver DRV 003 Mr Tariq Ali 01/05/2021 30/04/2022 Combined Driver DRV 005 Mr James Currie 01/04/2021 31/03/2022 Combined Driver DRV 007 Mr Mohammed Rhaoof Fazal 16/12/2020 30/04/2022 Private Hire Driver DRV 029 Mr Naveed Hussain 01/02/2020 31/01/2023 Hackney Carriage Driver DRV 030 Mr John Robert Fielding 01/04/2021 31/03/2022 Private Hire Driver DRV 033 Mr Mohammed Ali Saddique 01/03/2020 28/02/2023 Combined Driver DRV 037 Mr Tahir Ali 01/03/2020 28/02/2023 Private Hire Driver DRV 038 Mr Mohammed Razaq 01/10/2018 30/09/2021 Combined Driver DRV 041 Mr Saeed Bashir 01/12/2018 30/11/2021 Combined Driver DRV 044 Mr Lee Edwin Strange 01/06/2021 31/05/2022 Combined Driver DRV 049 Mr Zulfiqar Ali 01/12/2020 30/11/2023 Hackney Carriage Driver DRV 052 Mr Sabir Hussain 01/12/2020 30/11/2021 Combined Driver DRV 056 Mr Liaquat Ali 01/05/2019 30/04/2022 Combined Driver DRV 062 Mr Mohammed Ilyas 01/06/2019 31/05/2022 Combined Driver DRV 067 Mr Agha Abdul Salam Khan 01/03/2021 28/02/2022 Hackney Carriage Driver DRV 068 Mr Gordon Cutler 01/09/2020 31/08/2021 Hackney Carriage Driver DRV 069 Mr Anthony Keith Lea 01/03/2020 28/02/2023 Combined Driver DRV 071 Mr Anthony Michael Leeming 01/07/2021 30/06/2022 Combined Driver DRV 075 Mr Asif Mahmood 01/01/2020 -
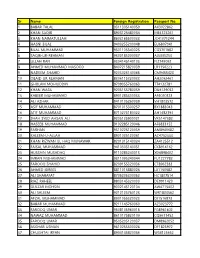
Sr Name Foreign Registration Passport No. 1 BABAR
Sr Name Foreign Registration Passport No. -

The Pakistan Army's Repression of the Punjab
Human Rights Watch July 2004 Vol. 16, No. 10 (C) Soiled Hands: The Pakistan Army’s Repression of the Punjab Farmers’ Movement Map 1: Pakistan – Provinces........................................................................................................ 1 Map 2: Punjab – Districts ............................................................................................................ 2 Table 1: Population Distribution across Okara District ......................................................... 3 I. Summary ..................................................................................................................................... 4 II. Key Recommendations........................................................................................................... 8 III. Background ............................................................................................................................. 9 Struggle Against Eviction ........................................................................................................ 9 “Ownership or Death”: Radicalization of the farmers’ movement ................................12 The Pakistan Rangers.............................................................................................................14 The Response of the Pakistan Army ...................................................................................17 IV. Human Rights Violations....................................................................................................19 Killings......................................................................................................................................20 -

Federal Service Tribunal, Islamabad. Cause List 03.12.2020 Thursday Court-I Before: Qazi Khalid Ali, Chairman and Mr
FEDERAL SERVICE TRIBUNAL, ISLAMABAD. CAUSE LIST 03.12.2020 THURSDAY COURT-I BEFORE: QAZI KHALID ALI, CHAIRMAN AND MR. MANZOOR ALI KHAN, MEMBER. S.No Name of Appellant Versus Appeal No. Nature Counsel for appellant Counsel for respondent Objections MISC.PETITION 1. QasimIftikhar NH &MP Mp. No. 308/2020 Implementation Mrs.Shireen Imran Deputy Attorney General Filed 1214(R)CS/2009 Of judgment 2. Ghulam Qadir NH &MP Mp. No. 309/2020 Implementation Mrs.Shireen Imran Deputy Attorney General Filed 80(L)CS/2008 Of judgment 3. Pervez Khan Pak. Railways MP.No.905/2019 Implementation Muhammad Owais Tajammal Hussain Not Filed 1927(R)CS/2018 of order 4. Muhammad Asif -do- MP.No.906/2019 -do- -do- -do- 1928(R)CS/2018 5. Afzal Hussain -do- MP.No.907/2019 -do- -do- -do- 1930(R)CS/2018 6. Muhammad Rafique Pak. Railways MP.No.404/2019 Implementation GhulamRasool Bhatti TajammalHussain Filed R-2/ 724(P)CS/2013 of Judgment Rejoinder 7. Muhammad Hanif -do- MP.No.405/2019 -do- -do- -do- 726(P)CS/2013 8. Gul Aslam Khan Post Office MP.No.194/2020 Implementation Muhammad Ramzan Khan Assistant Attorney General Not filed 901(P)CS/2017 of Judgment FOR FILING OF OBJECTIONS 9. Akhtar Hussain DIG Railways 507(R)CS/2020 Interim Relief Ghulam Rasool Bhatti Ms.Huma Noreen Hassan Not filed Police MP.No.816/2020 Accommodation 10. Abid Ali DIG Railways 508(R)CS/2020 -do- -do- Ms.Huma Noreen Hassan -do- Police MP.No.817/2020 Remanded case ( pre-admission ) 11. Asghar Ali Bhutto M/o Railways 2743(R)CS/2017 Reversion Muhammad Owais Muhammad Anwar Mughal Filed MP.No.3097/2017 MP.No.3101/2017 12. -

List of Directors PICG.Xlsx
Pakistan Institute of Corporate Governance – PICG List of Directors Training Program NO. NAMES DESIGNATION COMPANY YEAR 1 Mr. Bazl Khan Chairman IGI Funds Limited 2007 2 Mr. Ali Azam Shirazee CEO IGI Funds Limited 2007 3 Mr. Hasanali Abdullah Joint MD EFU General Insurance Ltd 2007 4 Mr. Abdul Aziz Yousuf Director Gul Ahmed Textile Mills Ltd 2007 5 Mr. Iqbal AliMohammed Chairman / Director MYBANK Limited 2007 6 Mr. Mohammad Hanif Jakhura CEO Central Depository Company of Pakistan 2007 7 Mr. Kamran Ahmed Qazi CFO & Co. Secretary Central Depository Company of Pakistan 2007 8 Mr. Riyaz T. Chinoy Chief Operating Officer International Industries Ltd 2007 9 Mr. Tameez-ul-Haque Company Secretary Adamjee Insurance Company Limited 2007 10 Ms. Neelofar Hameed Company Secretary International Industries Limited 2007 11 Mr. Fuzail Abbas CFO & SEVP Habib Metropolitan Bank Ltd 2007 12 Mr. Ekhlaq Ahmed EVP / Secretary National Bank of Pakistan 2007 13 Mr. Zafar Hussain Memon Director M. Yousuf Adil Saleem & CO. 2007 14 Mr. Aleem Ahmed Dani Group Director Finance Dawood Hercules Chemicals Ltd 2007 15 Mr. Abdul Samad Dawood CEO Dawood Corporation (Pvt) Ltd. 2007 16 Mr. Shahid Mahmood Dir Finance & Company Secretary KSB Pumps Co. Ltd 2007 Pakistan Institute of Corporate Governance – PICG List of Directors Training Program NO. NAMES DESIGNATION COMPANY YEAR 17 Syed Muhannad Ali Zamin SVP National Bank of Pakistan 2007 18 Mr. Moiz Ahmad Executive Director ICAP 2007 19 Ms. Sadia Khan Executive Director Delta Shipping (Pvt) Ltd 2007 20 Mr. Kaiser Naseem Manager PCG IFC 2007 M. Aslam & Company Chartered 21 Mr. Mohammed Aslam Principal 2007 Accountants 22 Mr. -

Abid Ali Abid - Poems
Classic Poetry Series Abid Ali Abid - poems - Publication Date: 2012 Publisher: Poemhunter.com - The World's Poetry Archive Abid Ali Abid(17 September 1906 - 20 January 1971.) Syed Abid Ali Abid (Urdu: ??? ???? ??? ????), M.A., L.L.B. , Urdu and Persian, poet, former principal Dyal Singh College, Lahore, Pakistan was born on 17 September 1906 at Dera Ismail Khan, British India and died in Lahore, Pakistan on 20 January 1971. <b> Life </b> He was well known for his literary criticism and had hundreds of students, many of them now themselves poets and writers. He wrote many books on literary criticism in Urdu and Persian. He also initiated and edited several literary journals, one of which is Sahifa-Lahore. He also was one of the initial drama writers and feature writers at the newly established Radio Pakistan Lahore in the late 1940s and 1950s. He was one of the story and dialogue writers of the first sound film (talking film) of the Punjab Heer Ranjha (1931). He survived 3 heart attacks but succumbed to the 4th. <b> Family </b> He had 3 more brothers besides him,as well as 4 brothers were Farzand Ali, Jawad Haider Nackvi and Mehmood Haider. The sisters were Zara, Mehmooda, Syeda and the youngest amongst all siblings was Shamim. One of his daughters, Shabnam Shakeel followed the footsteps of her father, and is a famous poetess, currently based in I only son Syed Menoo Chehr served as Secretary to Government of the Punjab in various departments as well as Chief settlement Commissioner,Punjab and was retired as Member Board of Revenue,Punjab and is also the author of two books titled "Mere Shab-o-Roz" and "karwan-e- Guzran".His two more books are in the process of publication .A novel titled "azmaish dil-o-nazar ki" and collection of short stories "roop behroop".He is also writing pen sketches of pominent personalities and his close friends..He practises as lawyer high cout.