Gramineae) in the Southeastern United States Gordon C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Additional Information
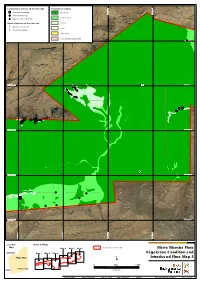
Current Survey Introduced Flora Records Vegetation Condition *Acetosa vesicaria Excellent 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 mE 535,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 536,000 mE 536,000 537,000 mE 537,000 534,000 mE 534,000 mE 535,000 534,000 mE 534,000 -

Wiregrass (Aristida Stricta)
Wiregrass (Aristida stricta) For definitions of botanical terms, visit en.wikipedia.org/wiki/Glossary_of_botanical_terms. Wiregrass is a perennial bunchgrass found in scrub, pinelands and coastal uplands throughout much of Florida. It is the dominant groundcover species in longleaf pine savannas and is a primary food source for gopher tortoises. Birds and small wildlife eat the seeds. Historically, cattle grazed on Wiregrass’s tender new growth. Wiregrass flowers are tiny and brown. They are born on spikelike terminal panicles Flower stalks are elongated and extend above the leaves. Leaf blades are long, thin and rolled inward, giving them a wiry appearance (hence the common name). They are erect and green when young Photo courtesy of Alan Cressler, Lady Bird Johnson Wildflower Center and begin to arch and turn brown as they age. Its fruits are small, yellowish caryopses. Seeds may be dispersed by wind, gravity or on the fur of passing animals. The genus name Aristida is from the Latin arista, meaning “awn” and referring to the three awns or bristle-like structures that extend from the florets. (An alternative common name is Pineland threeawn.) The species epithet, stricta, is from the Latin strictus, meaning straight or erect. Family: Poaceae (Grass family) Native range: Nearly throughout To see where natural populations of Wiregrass have been vouchered, visit www.florida.plantatlas.usf.edu. Hardiness: Zones 8A–10B Lifespan: Perennial Soil: Moist to dry, well-drained sandy soils Exposure: Full sun to partial shade Growth habit: 1–3’+ tall and equally wide Propagation: Division, seed Garden tips: Wiregrass is fast-growing and tolerant of drought conditions and low-nutrient soils. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Department of the Interior Fish and Wildlife Service
Tuesday, February 10, 2009 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Determination of Endangered Status for Reticulated Flatwoods Salamander; Designation of Critical Habitat for Frosted Flatwoods Salamander and Reticulated Flatwoods Salamander; Final Rule VerDate Nov<24>2008 14:17 Feb 09, 2009 Jkt 217001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10FER2.SGM 10FER2 erowe on PROD1PC63 with RULES_2 6700 Federal Register / Vol. 74, No. 26 / Tuesday, February 10, 2009 / Rules and Regulations DEPARTMENT OF THE INTERIOR during normal business hours, at U.S. Register on or before July 30, 2008, with Fish and Wildlife Service, Mississippi the final critical habitat rule to be Fish and Wildlife Service Fish and Wildlife Office, 6578 Dogwood submitted for publication in the Federal View Parkway, Jackson, MS 39213. Register by January 30, 2009. The 50 CFR Part 17 FOR FURTHER INFORMATION CONTACT: Ray revised proposed rule was signed on [FWS–R4–ES–2008–0082; MO 9921050083– Aycock, Field Supervisor, U.S. Fish and and delivered to the Federal Register on B2] Wildlife Service, Mississippi Field July 30, 2008, and it subsequently Office, 6578 Dogwood View Parkway, published on August 13, 2008 (73 FR RIN 1018–AU85 Jackson, MS 39213; telephone: 601– 47258). We also published supplemental information on the Endangered and Threatened Wildlife 321–1122; facsimile: 601–965–4340. If proposed rule to maintain the status of and Plants; Determination of you use a telecommunications device the frosted flatwoods salamander as Endangered Status for Reticulated for the deaf (TDD), call the Federal Information Relay Service (FIRS) at threatened (73 FR 54125; September 18, Flatwoods Salamander; Designation of 2008). -

Chapter 5 Phylogeny of Poaceae Based on Matk Gene Sequences
Chapter 5 Phylogeny of Poaceae Based on matK Gene Sequences 5.1 Introduction Phylogenetic reconstruction in the Poaceae began early in this century with proposed evolutionary hypotheses based on assessment of existing knowledge of grasses (e.g., Bew, 1929; Hubbard 1948; Prat, 1960; Stebbins, 1956, 1982; Clayton, 1981; Tsvelev, 1983). Imperical approaches to phylogenetic reconstruction of the Poaceae followed those initial hypotheses, starting with cladistic analyses of morphological and anatomical characters (Kellogg and Campbell, 1987; Baum, 1987; Kellogg and Watson, 1993). More recently, molecular information has provided the basis for phylogenetic hypotheses in grasses at the subfamily and tribe levels (Table 5.1). These molecular studies were based on information from chloroplast DNA (cpDNA) restriction sites and DNA sequencing of the rbcL, ndhF, rps4, 18S and 26S ribosomal DNA (rDNA), phytochrome genes, and the ITS region (Hamby and Zimmer, 1988; Doebley et al., 1990; Davis and Soreng, 1993; Cummings, King, and Kellogg, 1994; Hsiao et al., 1994; Nadot, Bajon, and Lejeune, 1994; Barker, Linder, and Harley, 1995; Clark, Zhang, and Wendel, 1995; Duvall and Morton, 1996; Liang and Hilu, 1996; Mathews and Sharrock, 1996). Although these studies have refined our concept of grass evolution at the subfamily level and, to a certain degree, at the tribal level, major disagreements and questions remain to be addressed. Outstanding discrepancies at the subfamily level include: 1) Are the pooids, bambusoids senso lato, or herbaceous bamboos the -

Who's Related to Whom?
149 Who’s related to whom? Recent results from molecular systematic studies Elizabeth A Kellogg Similarities among model systems can lead to generalizations systematist’s question-why are there so many different about plants, but understanding the differences requires kinds of organisms? Studies of the evolution of develop- systematic data. Molecular phylogenetic analyses produce ment demand that the investigator go beyond the model results similar to traditional classifications in the grasses system and learn the pattern of variation in its relatives (Poaceae), and relationships among the cereal crops [3*]. This requires a reasonable assessment of the relatives’ are quite clear. Chloroplast-based phylogenies for the identity. Solanaceae show that tomato is best considered as a species of Solarium, closely related to potatoes. Traditional Knowledge of plant relationships has increased rapidly classifications in the Brassicaceae are misleading with in the past decade, reflecting partly the development regard to true phylogenetic relationships and data are only of molecular systematics. It has been known for some now beginning to clarify the situation. Molecular data are time that plant classifications do not reflect phylogeny also being used to revise our view of relationships among accurately, even though both phylogeny and classification flowering plant families. Phylogenetic data are critical for are hierarchical. The hierarchy of classification was interpreting hypotheses of the evolution of development. imposed in the late 18th century, well before ideas of descent with modification (evolution) were prevalent [4]. These pre-evolutionary groups were then re-interpreted in Address an evolutionary context, and were assumed to be products Harvard University Herbaria, 22 Divinity Avenue Cambridge, MA of evolution, rather than man-made artefacts. -

Ornamental Grasses for the Midsouth Landscape
Ornamental Grasses for the Midsouth Landscape Ornamental grasses with their variety of form, may seem similar, grasses vary greatly, ranging from cool color, texture, and size add diversity and dimension to season to warm season grasses, from woody to herbaceous, a landscape. Not many other groups of plants can boast and from annuals to long-lived perennials. attractiveness during practically all seasons. The only time This variation has resulted in five recognized they could be considered not to contribute to the beauty of subfamilies within Poaceae. They are Arundinoideae, the landscape is the few weeks in the early spring between a unique mix of woody and herbaceous grass species; cutting back the old growth of the warm-season grasses Bambusoideae, the bamboos; Chloridoideae, warm- until the sprouting of new growth. From their emergence season herbaceous grasses; Panicoideae, also warm-season in the spring through winter, warm-season ornamental herbaceous grasses; and Pooideae, a cool-season subfamily. grasses add drama, grace, and motion to the landscape Their habitats also vary. Grasses are found across the unlike any other plants. globe, including in Antarctica. They have a strong presence One of the unique and desirable contributions in prairies, like those in the Great Plains, and savannas, like ornamental grasses make to the landscape is their sound. those in southern Africa. It is important to recognize these Anyone who has ever been in a pine forest on a windy day natural characteristics when using grasses for ornament, is aware of the ethereal music of wind against pine foliage. since they determine adaptability and management within The effect varies with the strength of the wind and the a landscape or region, as well as invasive potential. -

Viruses Virus Diseases Poaceae(Gramineae)
Viruses and virus diseases of Poaceae (Gramineae) Viruses The Poaceae are one of the most important plant families in terms of the number of species, worldwide distribution, ecosystems and as ingredients of human and animal food. It is not surprising that they support many parasites including and more than 100 severely pathogenic virus species, of which new ones are being virus diseases regularly described. This book results from the contributions of 150 well-known specialists and presents of for the first time an in-depth look at all the viruses (including the retrotransposons) Poaceae(Gramineae) infesting one plant family. Ta xonomic and agronomic descriptions of the Poaceae are presented, followed by data on molecular and biological characteristics of the viruses and descriptions up to species level. Virus diseases of field grasses (barley, maize, rice, rye, sorghum, sugarcane, triticale and wheats), forage, ornamental, aromatic, wild and lawn Gramineae are largely described and illustrated (32 colour plates). A detailed index Sciences de la vie e) of viruses and taxonomic lists will help readers in their search for information. Foreworded by Marc Van Regenmortel, this book is essential for anyone with an interest in plant pathology especially plant virology, entomology, breeding minea and forecasting. Agronomists will also find this book invaluable. ra The book was coordinated by Hervé Lapierre, previously a researcher at the Institut H. Lapierre, P.-A. Signoret, editors National de la Recherche Agronomique (Versailles-France) and Pierre A. Signoret emeritus eae (G professor and formerly head of the plant pathology department at Ecole Nationale Supérieure ac Agronomique (Montpellier-France). Both have worked from the late 1960’s on virus diseases Po of Poaceae . -

Large Trees, Supertrees, and Diversification of the Grass Family Trevor R
Aliso: A Journal of Systematic and Evolutionary Botany Volume 23 | Issue 1 Article 19 2007 Large Trees, Supertrees, and Diversification of the Grass Family Trevor R. Hodkinson Trinity College, Dublin, Ireland Nicolas Salamin University of Lausanne, Lausanne, Switzerland Mark W. Chase Royal Botanic Gardens, Kew, UK Yanis Bouchenak-Khelladi Trinity College, Dublin, Ireland Stephen A. Renvoize Royal Botanic Gardens, Kew, UK See next page for additional authors Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Hodkinson, Trevor R.; Salamin, Nicolas; Chase, Mark W.; Bouchenak-Khelladi, Yanis; Renvoize, Stephen A.; and Savolainen, Vincent (2007) "Large Trees, Supertrees, and Diversification of the Grass Family," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 23: Iss. 1, Article 19. Available at: http://scholarship.claremont.edu/aliso/vol23/iss1/19 Large Trees, Supertrees, and Diversification of the Grass Family Authors Trevor R. Hodkinson, Nicolas Salamin, Mark W. Chase, Yanis Bouchenak-Khelladi, Stephen A. Renvoize, and Vincent Savolainen This article is available in Aliso: A Journal of Systematic and Evolutionary Botany: http://scholarship.claremont.edu/aliso/vol23/iss1/ 19 Aliso 23, pp. 248–258 ᭧ 2007, Rancho Santa Ana Botanic Garden LARGE TREES, SUPERTREES, AND DIVERSIFICATION OF THE GRASS FAMILY TREVOR R. HODKINSON,1,5 NICOLAS SALAMIN,2 MARK W. CHASE,3 YANIS BOUCHENAK-KHELLADI,1,3 STEPHEN A. RENVOIZE,4 -

L. Watson – Publications 24 November 2016 1962 Watson, L
L. Watson – publications 24 November 2016 1962 Watson, L. (1962).The taxonomic significance of stomatal distribution and morphology in Epacridaceae. New Phytol. 61, 36–40. Watson, L. (1962). A peculiar Ericalean pollen grain. Nature 194, 889. Watson, L. (1962). A taxonomic revision of the genus Andersonia R.Br. (Epacridaceae). Kew Bull. 16, 85–127. 1963 Franks, J.W. and Watson, L. (1963). The pollen morphology of some critical Ericales. Pollen et Spores 5, 51–68. Watson, L. (1964). Some remarkable inflorescences in the Ericales and their taxonomic significance. Annals of Botany 28, 311-318. 1964 Watson, L. (1964). The taxonomic significance of certain anatomical observations in Ericaceae: the Ericoideae, Calluna and Cassiope. New Phytol. 63, 274–280. 1965 Drury, D.G. and Watson, L. (1965). Anatomy and the taxonomic significance of vegetative morphology in Senecio. New Phytol. 64, 307–314. Watson, L. (1965). The taxonomic implications of some anatomical variations among Ericaceae. J. Linn. Soc. Bot. 59, 111–125. 1966 Drury, D.G. and Watson, L. (1966). Taxonomic implications of a comparative anatomical study of Inuloideae-Compositae. Amer. J. Bot. 53, 828–833. Drury, D.G and Watson, L. (1966). Anatomy and the taxonomic significace of gross vegetative morphology in Senecio. New Phytol. 64, 307-314. Drury, D.G. and Watson, L.(1966). A bizarre pappus form in Senecio. Taxon 15, 309–311. Watson, L., Williams, W.T. and Lance, G.N. (1966). Angiosperm taxonomy: a comparative study of some novel numerical techniques. J. Linn. Soc. (Bot.) 59, 491–501. 1967 Watson, L., Williams, W.T. and Lance, G.N.