Reflection Measurements in IR Spectroscopy Author: Richard Spragg Perkinelmer, Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Diffuse Reflecting Power of Various Substances 1
. THE DIFFUSE REFLECTING POWER OF VARIOUS SUBSTANCES 1 By W. W. Coblentz CONTENTS Page I. Introduction . 283 II. Summary of previous investigations 288 III. Apparatus and methods 291 1 The thermopile 292 2. The hemispherical mirror 292 3. The optical system 294 4. Regions of the spectrum examined 297 '. 5. Corrections to observations 298 IV. Reflecting power of lampblack 301 V. Reflecting power of platinum black 305 VI. Reflecting power of green leaves 308 VII. Reflecting power of pigments 311 VIII. Reflecting power of miscellaneous substances 313 IX. Selective reflection and emission of white paints 315 X. Summary 318 Note I. —Variation of the specular reflecting power of silver with angle 319 of incidence I. INTRODUCTION In all radiometric work involving the measurement of radiant energy in absolute value it is necessary to use an instrument that intercepts or absorbs all the incident radiations; or if that is impracticable, it is necessary to know the amount that is not absorbed. The instruments used for intercepting and absorbing radiant energy are usually constructed in the form of conical- shaped cavities which are blackened with lampblack, the expecta- tion being that, after successive reflections within the cavity, the amount of energy lost by passing out through the opening is reduced to a negligible value. 1 This title is used in order to distinguish the reflection of matte surfaces from the (regular) reflection of polished surfaces. The paper gives also data on the specular reflection of polished silver for different angles of incidence, but it seemed unnecessary to include it in the title. -

Some Diffuse Reflection Problems in Radiation Aerodynamics Stephen Nathaniel Falken Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1964 Some diffuse reflection problems in radiation aerodynamics Stephen Nathaniel Falken Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Aerospace Engineering Commons Recommended Citation Falken, Stephen Nathaniel, "Some diffuse reflection problems in radiation aerodynamics " (1964). Retrospective Theses and Dissertations. 3848. https://lib.dr.iastate.edu/rtd/3848 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 65-4604 microfilmed exactly as received FALiKEN, Stephen Nathaniel, 1937- SOME DIFFUSE REFLECTION PROBLEMS IN RADIATION AERODYNAMICS. Iowa State University of Science and Technology Ph.D., 1964 Engineering, aeronautical University Microfilms, Inc., Ann Arbor, Michigan SOME DIFFUSE REFLECTIOU PROBLEMS IN RADIATION AERODYNAMICS "by Stephen Nathaniel Falken A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF HîILOSOEHï Major Subjects: Aerospace Engineering Mathematics Approved: Signature was redacted for privacy. Cmrg^Vf Major Work Signature was redacted for privacy. Heads of Mgjor Departments Signature was redacted for privacy. De of Gradu^ e College Iowa State University Of Science and Technology Ames, loTfa 196k ii TABLE OF CONTENTS page DEDICATION iii I. LIST OF SYMBOLS 1 II. INTRODUCTION. 5 HI. GENERAL AERODYNAMIC FORCE ANALYSIS 11 IV. THE THEORY OF RADIATION AERODYNAMICS Ik A. -

3 Specular Reflection Spectroscopy
3 Specular Reflection Spectroscopy Robert J. Lipert, Brian D. Lamp, and Marc D. Porter 3.1 INTRODUCTION This chapter on specular reflection spectroscopy focuses on applications in the mid-infrared region of the spectrum. Applications in other spectral regions or using other spectroscopic techniques, such as Raman spectros copy, generally follow similar patterns as discussed in this chapter. In the past few decades, infrared spectroscopy (IRS) has developed into an invaluable diagnostic tool for unraveling details about the bonding and molecular structure at surfaces [l, 2, 3, 4, 5, 6]. The importance of IRS derives from five major factors, The first and most critical factor is the role of interfacial phenomena in a vast number of emerging material and surface technologies. Examples include adhesion, catalysis, tribiology, microelec tronics, and electrochemistry [3, 7, 8, 9]. The second factor stems from the Modern Techniques in Applied Molecular Spectroscopy, Edited by Francis M. Mirabella. Techniques in Analytical Chemistry Series. ISBN 0-471-12359-5 © 1998 John Wiley & Sons, Inc. 83 84 Specular Reflection Spectroscopy information-rich content of an IRS spectrum of a surface. That is, the frequencies of the spectroscopic features can be used to identify the chemical composition of a surface and the magnitudes and polarization dependencies of the features used to determine average structural orientations. The third factor arises from advances in the performance of IRS instrumentation. The most important of these advancements are the high throughput and multi plex advantages of Fourier transform interferometry, the development of high sensitivity, low-noise IR detectors, and the improvement in the com putational rates of personal computers and their adaptation to the oper ation of chemical instrumentation. -

Ap Physics 2 Summary Chapter 21 – Reflection and Refraction
AP PHYSICS 2 SUMMARY CHAPTER 21 – REFLECTION AND REFRACTION . Light sources and light rays Light Bulbs, candles, and the Sun are examples of extended sources that emit light. Light from such sources illuminates other objects, which reflect the light. We see an object because incident light reflects off of it and reaches our eyes. We represent the travel of light rays (drawn as lines and arrows). Each point of a shining object or a reflecting object send rays in all directions. Law of reflection When a ray strikes a smooth surface such as a mirror, the angle between the incident ray and the normal line perpendicular to the surface equals the angle between the reflected ray and the normal line (the angle of incidence equals the angle of reflection). This phenomenon is called specular reflection. Diffuse reflection If light is incident on an irregular surface, the incident light is reflected in many different directions. This phenomenon is called diffuse reflection. Refraction If the direction of travel of light changes as it moves from one medium to another, the light is said to refract (bend) as it moves between the media. Snell’s law Light going from a lower to a higher index of refraction will bend toward the normal, but going from a higher to a lower index of refraction it will bend away from the normal. Total internal reflection If light tries to move from a more optically dense medium 1 of refractive index n1 into a less optically dense medium 2 of refractive index n2 (n1>n2), the refracted light in medium 2 bends away from the normal line. -

Method for Measuring Solar Reflectance of Retroreflective Materials Using Emitting-Receiving Optical Fiber
Method for Measuring Solar Reflectance of Retroreflective Materials Using Emitting-Receiving Optical Fiber HiroyukiIyota*, HidekiSakai, Kazuo Emura, orio Igawa, Hideya Shimada and obuya ishimura, Osaka City University Osaka, Japan *Corresponding author email: [email protected] ABSTRACT The heat generated by reflected sunlight from buildings to surrounding structures or pedestrians can be reduced by using retroreflective materials as building exteriors. However, it is very difficult to evaluate the solar reflective performance of retroreflective materials because retroreflective lightcannotbe determined directly using the integrating sphere measurement. To solve this difficulty, we proposed a simple method for retroreflectance measurementthatcan be used practically. A prototype of a specialapparatus was manufactured; this apparatus contains an emitting-receiving optical fiber and spectrometers for both the visible and the infrared bands. The retroreflectances of several types of retroreflective materials are measured using this apparatus. The measured values correlate well with the retroreflectances obtained by an accurate (but tedious) measurement. The characteristics of several types of retroreflective sheets are investigated. Introduction Among the various reflective characteristics, retroreflective materials as shown in Fig.1(c) have been widely used in road signs or work clothes to improve nighttime visibility. Retroreflection was given by some mechanisms:prisms, glass beads, and so on. The reflective performance has been evaluated from the viewpointof these usages only. On the other hand, we have shown thatretroreflective materials reduce the heatgenerated by reflected sunlight(Sakai, in submission). The use of such materials on building exteriors may help reduce the urban heatisland effect. However, itis difficultto evaluate the solar reflective performance of retroreflective materials because retroreflectance cannot be determined using Figure 1. -

FTIR Reflection Techniques
FT-IR Reflection Techniques Vladimír Setnička Overview – Main Principles of Reflection Techniques Internal Reflection External Reflection Summary Differences Between Transmission and Reflection FT-IR Techniques Transmission: • Excellent for solids, liquids and gases • The reference method for quantitative analysis • Sample preparation can be difficult Reflection: • Collect light reflected from an interface air/sample, solid/sample, liquid/sample • Analyze liquids, solids, gels or coatings • Minimal sample preparation • Convenient for qualitative analysis, frequently used for quantitative analysis FT-IR Reflection Techniques Internal Reflection Spectroscopy: Attenuated Total Reflection (ATR) External Reflection Spectroscopy: Specular Reflection (smooth surfaces) Combination of Internal and External Reflection: Diffuse Reflection (DRIFTs) (rough surfaces) FT-IR Reflection Techniques • Infrared beam reflects from a interface via total internal reflectance • Sample must be in optical contact with the crystal • Collected information is from the surface • Solids and powders, diluted in a IR transparent matrix if needed • Information provided is from the bulk matrix • Sample must be reflective or on a reflective surface • Information provided is from the thin layers Attenuated Total Reflection (ATR) - introduced in the 1960s, now widely used - light introduced into a suitable prism at an angle exceeding the critical angle for internal reflection an evanescent wave at the reflecting surface • sample in close contact Single Bounce ATR with IRE -

Reflectance IR Spectroscopy, Khoshhesab
11 Reflectance IR Spectroscopy Zahra Monsef Khoshhesab Payame Noor University Department of Chemistry Iran 1. Introduction Infrared spectroscopy is study of the interaction of radiation with molecular vibrations which can be used for a wide range of sample types either in bulk or in microscopic amounts over a wide range of temperatures and physical states. As was discussed in the previous chapters, an infrared spectrum is commonly obtained by passing infrared radiation through a sample and determining what fraction of the incident radiation is absorbed at a particular energy (the energy at which any peak in an absorption spectrum appears corresponds to the frequency of a vibration of a part of a sample molecule). Aside from the conventional IR spectroscopy of measuring light transmitted from the sample, the reflection IR spectroscopy was developed using combination of IR spectroscopy with reflection theories. In the reflection spectroscopy techniques, the absorption properties of a sample can be extracted from the reflected light. Reflectance techniques may be used for samples that are difficult to analyze by the conventional transmittance method. In all, reflectance techniques can be divided into two categories: internal reflection and external reflection. In internal reflection method, interaction of the electromagnetic radiation on the interface between the sample and a medium with a higher refraction index is studied, while external reflectance techniques arise from the radiation reflected from the sample surface. External reflection covers two different types of reflection: specular (regular) reflection and diffuse reflection. The former usually associated with reflection from smooth, polished surfaces like mirror, and the latter associated with the reflection from rough surfaces. -

Emission and Reflection from the Ocean and Land Surfaces 1
Lecture 5 Emission and reflection from the ocean and land surfaces Objectives: 1. Interaction of radiation with the surfaces. 2. Emission from the ocean and land surfaces. 3. Reflection from the ocean and land surfaces. Required reading: G: 4.3, 4.4, 4.5 Additional reading: CNES online tutorial Chapters 5-6 http://ceos.cnes.fr:8100/cdrom-00/ceos1/science/baphygb/intro/content.htm 1. Interaction of radiation and the surfaces. The ocean and land surfaces can modify the atmospheric radiation field by a) reflecting a portion of the incident radiation back into the atmosphere; b) transmitting some incident radiation; c) absorbing a portion of incident radiation (see Lecture 4, Kirchhoff’s law); d) emitting the thermal radiation (see Lecture 4, Kirchhoff’s law); INCIDENT RADIATION REFLECTED RADIATION ABSORBED RADIATION RANSMITTED RADIATION TRANSMITTED RADIATION 1 Conservation of energy requires that monochromatic radiation incident upon any surface, Ii, is either reflected, Ir, absorbed, Ia, or transmitted, It . Thus Ii = Ir + Ia + It [5.1] 1 = Ir / Ii + Ia / Ii + It / Ii = R + A+ T [5.2] where T is the transmission, A is the absorption, and R is the reflection of the surface. In general, T, A, and R are a function of the wavelength: Rλ + Aλ+ Tλ =1 [5.3] Blackbody surfaces (no reflection) and surfaces in LTE (from Kirchhoff’s law): Aλ = ελ [5.4] Opaque surfaces (no transmission): Rλ + Aλ = 1 [5.5] Thus for the opaque surfaces ελ = 1- Rλ [5.6] 2. Emission from the ocean and land surfaces. Emissivity of the surfaces: • In general, emissivity depends on the direction of emission, surface temperature, wavelength and some physical properties of the surface • In the thermal IR (4µm<λ< 100µm), nearly all surfaces are efficient emitters with the emissivity > 0.8 and their emissivity does not depend on the direction. -

Reflection and Refraction
Fi Reflection and A thi Refraction wa en at th los Th hen you shine a beam of light on a mir- ref rot the light doesn't travel through the dii Wmurmur. but a returned by the mirror's mutate bact into the ad Aben sound aases strike a canyon wall. they return to you as an echo. When a transverse wave transmitted along Changes tn lasaht speed produce a spring reaches a wall. it rrverNes direction In all these situations. refraction. waves remain in one medium rather than enter a new medium. These waves are reflected. In other situations, as when light passes from air into water, waves travel from one medium into another. When waves strike the surface of a medium at an angle, their direction changes as they enter the second medium. These waves are refracted. This is evident when a pencil in a glass of water appears to be bent. Usually waves are partly reflected and partly refracted when they wa fall on a transparent medium. When light shines on water, for exam- refl ple, some of the light is reflected and some is refracted. To under- pet stand this, let's see how reflection occurs. refl dic the 29.1 Reflection When a wave reaches a boundary between two media, some or all of the wave bounces back into the first medium. This is reflection. 21 For example, suppose you fasten a spring to a wall and send a pulse along the spring's length (Figure 29.1). The wall is a very rigid In medium compared with the spring. -
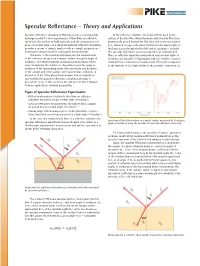
Specular Reflectance – Theory and Applications
Specular Reflectance – Theory and Applications Specular reflectance sampling in FTIR represents a very important At the reflective substrate, the beam reflects back to the technique useful for the measurement of thin films on reflective surface of the thin film. When the beam exits the thin film it has substrates, the analysis of bulk materials and the measurement of geometrically passed through the film twice and is now represented mono-molecular layers on a substrate material. Often this technique as IA. Infrared energy is absorbed at characteristic wavelengths as provides a means of sample analysis with no sample preparation – this beam passes through the thin film and its spectrum is recorded. keeping the sample intact for subsequent measurements. The specular reflectance spectra produced from relatively thin The basics of the sampling technique involve measurement films on reflective substrates measured at near-normal angle of of the reflected energy from a sample surface at a given angle of incidence are typically of high quality and very similar to spectra incidence. The electromagnetic and physical phenomena which obtained from a transmission measurement. This result is expected occur at and near the surface are dependent upon the angle of as the intensity of IA is high relative to the specular component (IR ). incidence of the illuminating beam, refractive index and thickness of the sample and other sample and experimental conditions. A discussion of all of the physical parameters and considerations surrounding the specular reflectance -

Chapter 22 Reflection and Refraction of Light Wavelength the Distance Between Any Two Crests of the Wave Is Defined As the Wavel
Chapter 22 Reflection and Refraction of Light Wavelength Dual Nature of Light The distance between any two crests of the • Experiments can be devised that will wave is defined as the wavelength display either the wave nature or the particle nature of light • Nature prevents testing both qualities at the same time Geometric Optics – Using a Ray The Nature of Light Approximation • “Particles” of light are called photons • Light travels in a straight-line path in a • Each photon has a particular energy homogeneous medium until it –E = h ƒ encounters a boundary between two – h is Planck’s constant different media • h = 6.63 x 10-34 J s •The ray approximation is used to – Encompasses both natures of light represent beams of light • Interacts like a particle •A ray of light is an imaginary line drawn • Has a given frequency like a wave along the direction of travel of the light beams Ray Approximation Geometric Optics •A wave front is a surface passing through points of a wave that have the same phase and amplitude • The rays, corresponding to the direction of the wave motion, are perpendicular to the wave fronts Reflection QUICK QUIZ 22.1 Diffuse refection: The objects has irregularities that spread out an initially parallel beam of Which part of the figure below shows specular reflection of light in all directions to produce light from the roadway? diffuse reflection Specular reflection (mirror): When a parallel beam of light is directed at a smooth surface, it is specularly reflected in only one direction. The color of an object we see depends on two things: Law of Reflection The angle of incidence = the angle of reflection The kind of light falling on it and nature of its surface For instance, if white light is used to illuminate an object that absorbs all color other than red, the object will appear red. -

Specular Reflection on Titan: Liquids in Kraken Mare Katrin Stephan,1 Ralf Jaumann,1,2 Robert H
GEOPHYSICAL RESEARCH LETTERS, VOL. 37, L07104, doi:10.1029/2009GL042312, 2010 Click Here for Full Article Specular reflection on Titan: Liquids in Kraken Mare Katrin Stephan,1 Ralf Jaumann,1,2 Robert H. Brown,3 Jason M. Soderblom,3 Laurence A. Soderblom,4 Jason W. Barnes,5 Christophe Sotin,6 Caitlin A. Griffith,3 Randolph L. Kirk,4 Kevin H. Baines,6 Bonnie J. Buratti,6 Roger N. Clark,7 Dyer M. Lytle,3 Robert M. Nelson,6 and Phillip D. Nicholson8 Received 4 January 2010; revised 22 February 2010; accepted 25 February 2010; published 7 April 2010. [1] After more than 50 close flybys of Titan by the Cassini reflections results either because the putative lakes are not spacecraft, it has become evident that features similar in filled with liquids or the lakes have not been observed in morphology to terrestrial lakes and seas exist in Titan’s specular geometry. A further impediment is that the northern polar regions. As Titan progresses into northern spring, polar region, where several extensive features exists, including the much more numerous and larger lakes and seas in the the more than 1000 km wide Kraken Mare [Stofan et al., north‐polar region suggested by Cassini RADAR data, are 2007; Turtle et al., 2009], was not illuminated during Titan’s becoming directly illuminated for the first time since the long winter. Nevertheless, the northern polar region is now arrival of the Cassini spacecraft. This allows the Cassini illuminated for the first time since Cassini arrived at Saturn in optical instruments to search for specular reflections to 2004, thus searches for specular reflections from northern provide further confirmation that liquids are present in bodies of liquid on Titan are now possible, with our first these evident lakes.