Monte Carlo Simulation Applied to Uncertainties in Iodine-123 Assay And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Huidige Situatie En Toekomstverkenning
Stichting Laka: Documentatie- en onderzoekscentrum kernenergie De Laka-bibliotheek The Laka-library Dit is een pdf van één van de publicaties in This is a PDF from one of the publications de bibliotheek van Stichting Laka, het in from the library of the Laka Foundation; the Amsterdam gevestigde documentatie- en Amsterdam-based documentation and onderzoekscentrum kernenergie. research centre on nuclear energy. Laka heeft een bibliotheek met ongeveer The Laka library consists of about 8,000 8000 boeken (waarvan een gedeelte dus ook books (of which a part is available as PDF), als pdf), duizenden kranten- en tijdschriften- thousands of newspaper clippings, hundreds artikelen, honderden tijdschriftentitels, of magazines, posters, video's and other posters, video’s en ander beeldmateriaal. material. Laka digitaliseert (oude) tijdschriften en Laka digitizes books and magazines from the boeken uit de internationale antikernenergie- international movement against nuclear beweging. power. De catalogus van de Laka-bibliotheek staat The catalogue of the Laka-library can be op onze site. De collectie bevat een grote found at our website. The collection also verzameling gedigitaliseerde tijdschriften uit contains a large number of digitized de Nederlandse antikernenergie-beweging en magazines from the Dutch anti-nuclear power een verzameling video's. movement and a video-section. Laka speelt met oa. haar informatie- Laka plays with, amongst others things, its voorziening een belangrijke rol in de information services, an important role in the Nederlandse anti-kernenergiebeweging. Dutch anti-nuclear movement. Appreciate our work? Feel free to make a small donation. Thank you. www.laka.org | [email protected] | Ketelhuisplein 43, 1054 RD Amsterdam | 020-6168294 | L.P. -

Viiith INTERNATIONAL SYMPOSIUM on NUCLEAR MEDICINE
INIS-mf--10949 VIIIth INTERNATIONAL SYMPOSIUM ON NUCLEAR MEDICINE JUNE 2 - 5, 1986 KARLOVY VARY CZECHOSLOVAKIA ABSTRACTS ABSTRACTS The booklet contains all abstracts of papers submitted in time to the Secretariat of the Symposium. The abstracts were reproduced from the original forms sent by the ithors. The responsibilit >r the contents and gram- matical style the. re lies upon the authors. 17 ANTIBODY GUIDED TUMOR DETECTION IN PATIENTS WITH CARCINOMA. P.Riva.G.Paganelli, C.Cacciaguerra,V.Tison5G.Landi, G. Riceputi,G.Moscatelli.M.Agostini. Istituto Oncologico Romagnolo. Servizio di Medicir.a Nucleare Ospedale M.Bufalini 47023 Cesena ITALY. As a unit of a multicenter clinical trial coordi - nated by the National Reserch Council we studied two groups of patients,in the last three years.In the first group of undred patients affected by ma- lignat melanoma with stage I-IV we were able to de tect the 75% of well documented lesions employing a 99mTC labelled F(ab')2 fragments of MoAb raised a- gainst melanoma (HMW MAA)225.28S(TECNEMAB 1-SORIN BIOMEDICA).In addition a certain number of unknown metastases were also detected and confirmed after- wards. The second group consisted of two-undred pa tients with gastro-intestinal,breast and lung carci^ noma.In these cases we used a 111 In and or 131 I labelled F(ab')i) fragments of a MoAb Raised against CEA(clone F023C5-S'?RIN-Biomedica) with best results in patients bearing colon retto carcinoma and lung cancer.More in detail we obtained the 8<~-% and the 91% of positive scans respectively.In order to im- prove the sensitivity and the tumour/BK ratio some patients with G.I. -

Does Your Patient Have Bile Acid Malabsorption?
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 Carol Rees Parrish, MS, RDN, Series Editor Does Your Patient Have Bile Acid Malabsorption? John K. DiBaise Bile acid malabsorption is a common but underrecognized cause of chronic watery diarrhea, resulting in an incorrect diagnosis in many patients and interfering and delaying proper treatment. In this review, the synthesis, enterohepatic circulation, and function of bile acids are briefly reviewed followed by a discussion of bile acid malabsorption. Diagnostic and treatment options are also provided. INTRODUCTION n 1967, diarrhea caused by bile acids was We will first describe bile acid synthesis and first recognized and described as cholerhetic enterohepatic circulation, followed by a discussion (‘promoting bile secretion by the liver’) of disorders causing bile acid malabsorption I 1 enteropathy. Despite more than 50 years since (BAM) including their diagnosis and treatment. the initial report, bile acid diarrhea remains an underrecognized and underappreciated cause of Bile Acid Synthesis chronic diarrhea. One report found that only 6% Bile acids are produced in the liver as end products of of British gastroenterologists investigate for bile cholesterol metabolism. Bile acid synthesis occurs acid malabsorption (BAM) as part of the first-line by two pathways: the classical (neutral) pathway testing in patients with chronic diarrhea, while 61% via microsomal cholesterol 7α-hydroxylase consider the diagnosis only in selected patients (CYP7A1), or the alternative (acidic) pathway via or not at all.2 As a consequence, many patients mitochondrial sterol 27-hydroxylase (CYP27A1). are diagnosed with other causes of diarrhea or The classical pathway, which is responsible for are considered to have irritable bowel syndrome 90-95% of bile acid synthesis in humans, begins (IBS) or functional diarrhea by exclusion, thereby with 7α-hydroxylation of cholesterol catalyzed interfering with and delaying proper treatment. -

Multicentre Prospective Survey of Sehcat Provision and Practice in the UK
BMJ Open Gastroenterol: first published as 10.1136/bmjgast-2016-000091 on 1 May 2016. Downloaded from Imaging Multicentre prospective survey of SeHCAT provision and practice in the UK Jennifer A Summers,1,2 Janet Peacock,1,2 Bolaji Coker,1,2 Viktoria McMillan,3,4 Mercy Ofuya,1,2 Cornelius Lewis,3,4 Stephen Keevil,3,5 Robert Logan,6 John McLaughlin,7,8 Fiona Reid,1,2 To cite: Summers JA, ABSTRACT et al Summary box Peacock J, Coker B, . Objective: A clinical diagnosis of bile acid Multicentre prospective malabsorption (BAM) can be confirmed using SeHCAT survey of SeHCAT provision What is already known about this subject? (tauroselcholic (75selenium) acid), a radiolabelled and practice in the UK. BMJ ▸ Bile acid malabsorption (BAM) is an established Open Gastro 2016;3: synthetic bile acid. However, while BAM can be the cause of chronic diarrhoea and results in signifi- e000091. doi:10.1136/ cause of chronic diarrhoea, it is often overlooked as a cantly adverse quality of life for affected individuals. bmjgast-2016-000091 potential diagnosis. Therefore, we investigated the use ▸ Diagnosis of BAM can be confirmed using of SeHCAT for diagnosis of BAM in UK hospitals. SeHCAT (tauroselcholic (75selenium) acid), a Design: A multicentre survey was conducted radiolabelled synthetic bile acid. However, this capturing centre and patient-level information detailing diagnostic tool is not consistently applied in patient care-pathways, clinical history, SeHCAT results, National Health Service (NHS) centres in the UK. Additional material is treatment with bile acid sequestrants (BAS), and ▸ Patients diagnosed with BAM can benefit from published online only. -

Northern Ireland Nuclear Medicine Equipment Survey, 2017
Northern Ireland nuclear medicine equipment survey 2017 Northern Ireland nuclear medicine equipment survey 2017 About Public Health England Public Health England exists to protect and improve the nation’s health and wellbeing and reduce health inequalities. We do this through world-leading science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. We are an executive agency of the Department of Health and Social Care, and a distinct delivery organisation with operational autonomy. We provide government, local government, the NHS, Parliament, industry and the public with evidence-based professional, scientific and delivery expertise and support. Public Health England Wellington House 133-155 Waterloo Road London SE1 8UG Tel: 020 7654 8000 www.gov.uk/phe Twitter: @PHE_uk Facebook: www.facebook.com/PublicHealthEngland © Crown copyright 2019 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v3.0. To view this licence, visit OGL. Where we have identified any third-party copyright information you will need to obtain permission from the copyright holders concerned. Published January 2019 PHE publications PHE supports the UN Sustainable Development Goals 2 Northern Ireland nuclear medicine equipment survey 2017 Contents Executive summary 4 Introduction 5 Methodology 6 Number of procedures performed 7 Procedures per scanner 8 Age of Scanners 9 Procedures reported for each organ or system 10 Procedures reported -

Abschlussbereicht Zum UFO-Plan Vorhaben 3617S42443
Ressortforschungsberichte zum Strahlenschutz Erhebung von Häufigkeit und Dosis nuklearmedizinischer Untersuchungsverfahren? - Vorhaben 3617S42443 Auftragnehmer: Städtisches Klinikum Braunschweig gGmbH M. Borowski L. Pirl Das Vorhaben wurde mit Mitteln des Bundesministeriums für Umwelt, Naturschutz und nukleare Sicherheit (BMU) und im Auftrag des Bundesamtes für Strahlenschutz (BfS) durchgeführt. Name Autor/Herausgeber Dieser Band enthält einen Ergebnisbericht eines vom Bundesamt für Strahlenschutz im Rahmen der Ressortforschung des BMU (Ressortforschungsplan) in Auftrag gegebenen Untersuchungsvorhabens. Verantwortlich für den Inhalt sind allein die Autoren. Das BfS übernimmt keine Gewähr für die Richtigkeit, die Genauigkeit und Vollständigkeit der Angaben sowie die Beachtung privater Rechte Dritter. Der Auftraggeber behält sich alle Rechte vor. Insbesondere darf dieser Bericht nur mit seiner Zustimmung ganz oder teilweise vervielfältigt werden. Der Bericht gibt die Auffassung und Meinung des Auftragnehmers wieder und muss nicht mit der des BfS übereinstimmen. BfS-RESFOR-164/20 Bitte beziehen Sie sich beim Zitieren dieses Dokumentes immer auf folgende URN: urn:nbn:de:0221-2020091722831 Salzgitter, September 2020 Abschlussbericht zum Ressortforschungsvorhaben 3617S42443 Erhebung von Häufigkeit und Dosis nuklearmedizinischer Untersuchungsverfahren Auftragnehmer: Städtisches Klinikum Braunschweig gGmbH Autoren: M. Borowski, L. Pirl Der Bericht gibt die Auffassung und Meinung des Auftragnehmers wieder und muss nicht mit der Meinung der Auftraggeberin -

First European Congress on Hereditary ATTR Amyloidosis Paris, France
Orphanet Journal of Rare Diseases 2015, Volume 10 Suppl 1 http://www.ojrd.com/content/10/S1/I1 MEETING ABSTRACTS Open Access First European Congress on Hereditary ATTR amyloidosis Paris, France. 2-3 November 2015 Published: 2 November 2015 These abstracts are available online at http://www.ojrd.com/supplements/10/S1 INVITED SPEAKER PRESENTATIONS Rice ASC, Rowbotham M, Sena E, Siddall P, Smith B, Wallace M: Pharmacotherapy for neuropathic pain in adults: systematic review, meta- Lancet Neurol I1 analysis and NeuPSIG recommendations. 2015, 14:162-73. Symptomatic therapy in ATTR amyloidosis: pain killers in TTR-FAP Nadine Attal I2 INSERM U-987, Centre dÂ’’Evaluation et de Traitement de la Douleur, CHU Neuropathic phenotypes and natural history of FAP Ambroise Parc APHP, F-92100 Boulogne-Billancourt, France and University David Adams Versailles Saint-Quentin, Versailles, F-78035, France Centre Paris-Sud, APHP, Hopital de Bicetre and Centre de Reference National E-mail: [email protected] des Neuropathies Amyloides Familiales, 94275 Le Kremlin-Bicetre, France Orphanet Journal of Rare Diseases 2015, 10(Suppl 1):I1 Orphanet Journal of Rare Diseases 2015, 10(Suppl 1):I2 Familial amyloidosis typically causes a nerve length-dependent small fiber TTR-FAP have been described more than 60 years ago by Corino Andrade polyneuropathy that starts in the feet with loss of temperature and pain in Porto (Brain, 1952). This peculiar disease affected many families with an sensations, associated with autonomic dysfunction, which can be extremely autosomal dominant transmission, in the third decade of life, characterized severe and life threatening. Neuropathic pain is commonly associated with by a progressive peripheral neuropathy starting in the lower extremities amyloid neuropathy. -

Use of the Sehcat Test in the Investigation of Diarrhoea
Postgrad Med J: first published as 10.1136/pgmj.68.798.272 on 1 April 1992. Downloaded from Postgrad Med J (1992) 68, 272 - 276 © The Fellowship of Postgraduate Medicine, 1992 Use ofthe SeHCAT test in the investigation ofdiarrhoea G.A. Ford*, J.D. Preece, I.H. Davies and S.P. Wilkinson Departments ofMedicine and Medical Physics, Gloucestershire Royal Hospital, Great Western Road, Gloucester GLI 3NN, UK Summary: The SeHCAT test was used to investigate possible bile acid malabsorption in 166 patients presenting to a district general hospital with chronic diarrhoea of uncertain cause. Eighty-four (51%) patients had impaired SeHCAT retention. These included 23 of 28 patients with a possible type I abnormality (terminal ileal resection or disease, previous pelvic radiotherapy), 20 of 74 with a possible type II abnormality (idiopathic diarrhoea), 32 of 45 with a possible type III abnormality (post- cholecystectomy, post-vagotomy), and 9 of 19 with diarrhoea associated with diabetes. Patients with severe bile acid malabsorption demonstrated a good response to cholestyramine whereas the response in patients with a mildly abnormal SeHCAT retention was variable. Bile acid malabsorption is an important cause of diarrhoea in patients presenting with unexplained chronic diarrhoea. Introduction Bile acid malabsorption is recognized as a cause of Patients and methods chronic diarrhoea.' In healthy individuals bile acids are reabsorbed by the terminal ileum. Bile One hundred and sixty-six patients (89 female) acids may induce diarrhoea if they enter the colon, aged 18-79 years were studied. Clinical details of copyright. through inhibition of water and electrolyte trans- all patients undergoing the SeHCAT test were port and possibly by increasing colonic motility.2`7 obtained from retrospective review of patient Three types of bile acid malabsorption have been records. -

British Nuclear Medicine Society 47 Annual Spring Meeting, Oxford 1 St
Abstracts British Nuclear Medicine Society 47th Annual Spring Meeting, Oxford 1st–3rd April 2019 Nuclear Medicine Communications 2019, 00:000–000 1. Imaging patients with possible cardiac sarcoidosis: aOxford University Hospitals NHS Foundation Trust, Review of initial experience Oxford, United Kingdom and bUniversity of Oxford, Oxford, Pamela Moyade and Sobhan Vinjamuri United Kingdom Royal Liverpool and Broadgreen University Hospitals Trust, Liverpool, United Kingdom The aim of the study was to investigate the threshold value of the estimated GFR (eGFR) which would predict Aim: To evaluate the adequacy of patient preparation for a measured GFR (mGFR) of ≤ 25 ml/min/1.73 m2 in order cardiac FDG scans and to assess concurrence between to determine if the patient requires a 24 h single blood blinded interpreters for image interpretation. sample (SS), following the new BNMS guidelines, or Materials and methods: 31 patients with clinical suspicion of alternatively use the slope intercept (SI) method to mea- cardiac sarcoidosis who underwent 18F-FDG PET/CT scans sure their GFR. Data from 1956 patient studies were used were retrospectively evaluated. All patients were instructed to calculate SI and SS values of the mGFR. A sub-data ≤ 2 to follow a high fat – low carbohydrate diet for 18 h, followed set of 241 patients with a mGFR of 80 ml/min/1.73 m by a water only fast for 18 h. The study was conducted only if and an eGFR measurement taken within 28 days was the patient’s blood glucose was <11.0. Each scan was inter- selected. preted separately by three blinded and experienced Nuclear ThereisalargevariationintheaccuracyofusingtheeGFR Medicine Physicians. -

Wo 2008/116165 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date PCT 25 September 2008 (25.09.2008) WO 2008/116165 A2 (51) International Patent Classification: MCNEIL, Laurie [US/US]; University Of North Car A61M 11/02 (2006.01) olina-Chapel Hill, Department Of Physics And Astronomy, Chapel Hill, NC (US). WETZEL, Paul [US/US]; 456 (21) International Application Number: Quail Hollow Road, Jefferson, NC 28640 (US). CRISS, PCT/US2008/057847 Ron [US/US]; P.O. Box 1598, West Jefferson, NC 28694 (US). (22) International Filing Date: 2 1 March 2008 (21.03.2008) (74) Agent: RISLEY, David, R.; Thomas, Kayden, Horste- (25) Filing Language: English meyer & Risley, LIp., 600 Galleria Parkway, Atlanta, GA 30339-5948 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, 11/689,315 21 March 2007 (2 1.03.2007) US CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, (71) Applicant (for all designated States except US): NEXT IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, SAFETY, INC. [US/US]; 1329 Phoenix Colvard Drive, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, Jefferson, NC 28640 (US). MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, (72) Inventors; and SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (75) Inventors/Applicants (for US only): LEMAHIEU, Ed¬ ZA, ZM, ZW ward [US/US]; 1120 Dean Avenue, San Jose, CA 95125 (US). -

CV Dott Stefano Panareo 2020
Curriculum Vitae Dott. Stefano Panareo Informazioni personali Nome Stefano Panareo Data di nascita 12 maggio 1970 (Ferrara) Residenza Telefono 0532 237458 (studio) 0532 236387 (segreteria) Nazionalità Italiana Fax 0532 237553 (segreteria) E-mail [email protected], PEC [email protected] Codice fiscale PNR SFN 70E 12D 548J Istruzione e formazione • Nome e tipo di istituto di istruzione o Liceo Classico “Ludovico Ariosto” di Ferrara formazione • Principali materie / abilità Materie di base previste dall'ordinamento scolastico professionali oggetto dello studio • Qualifica conseguita Diploma di Maturità Sperimentale Indirizzo Linguistico Moderno (1984 – 1989) • Nome e tipo di istituto di istruzione o Università degli Studi di Ferrara formazione • Principali materie / abilità Materie previste dall'ordinamento del Corso di Laurea in Medicina e Chirurgia professionali oggetto dello studio • Qualifica conseguita Diploma di Laurea in Medicina e Chirurgia (104/110) – data 28.03.1998 • Nome e tipo di istituto di istruzione o Università degli Studi di Ferrara formazione • Principali materie / abilità Materie previste dall'ordinamento del Corso di Laurea in Medicina e Chirurgia professionali oggetto dello studio • Qualifica conseguita Abilitazione all'esercizio della professione di medico-chirurgo (97/100) – data 11.1998 Iscrizione All’Albo professionale dei Medici Chirughi e Odnotoiatri di Ferrara (n° 7306) – data 12.1998 • Nome e tipo di istituto di istruzione o Università degli Studi di Ferrara formazione • Principali materie / abilità -
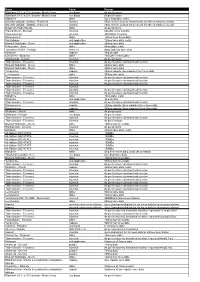
FOI 218-1314 Document 1
Name Form Dosage Riboflavin 0.1% in 20% dextran - Medio Cross eye drops As per literature Riboflavin 0.1% in 20% dextran - Medio Cross eye drops As per literature Midodrine tablet Up to 5mg daily, orally Dimethyl sulfoxide (DMSO) - Rimso-50 solution 50mL of 50% solution intravesically (for fifteen minutes), weekly Dimethyl sulfoxide (DMSO) - Rimso-50 solution 50mL of 50% solution intravesically (for fifteen minutes), weekly Buspirone Hydrochloride tablet As per protocol Ergocalciferol - Sterogyl injection 600,000 IU six monthly Cholecalciferol injection 300,000IU 3 monthly Paromomycin tablet 160mg three times daily Furazolidone oral application 100mg twice daily, orally Bismuth Subcitrate - Denol oral application 240mg twice daily Febuxostat - Uloric tablet 40mg daily, orally Tacrolimus 0.03% - Protopic ointment Apply topically twice daily Melatonin capsule 3mg at night Cinnarizine - Stugeron tablet 25mg three times daily Adalimumab - Humira injection As per protocol Triamcinolone - Triesence injection As per literature via intravitreal injection Bismuth Subcitrate - Denol tablet 240mg twice daily Bismuth Subcitrate - Denol tablet 240mg twice daily Tetracycline capsule 250mg capsule, two capsules four times daily Levofloxacin tablet 500mg twice daily Triamcinolone - Triesence injection As per literature via intravitreal injection Triamcinolone - Triesence injection As per literature via intravitreal injection Triamcinolone - Triesence injection As per literature via intravitreal injection Triamcinolone - Triesence injection Triamcinolone