Roosting Ecology of the Grey-Headed Flying Fox: Spatial Dispersion in a Summer Camp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Agathis Macrophylla Araucariaceae (Lindley) Masters
Agathis macrophylla (Lindley) Masters Araucariaceae LOCAL NAMES English (pacific kauri); Fijian (da‘ua,dakua dina,makadri,makadre,takua makadre,dakua,dakua makadre) BOTANIC DESCRIPTION Agathis macrophylla is a tall tree typically to about 30–40 m tall, 3 m in bole diameter, with a broad canopy of up to 36 m diameter. Branches may be erect to horizontal and massive. Mature specimens have wide, spreading root systems whereas seedlings and young specimens have a vigorous taproot with one or more whorls of lateral roots. Leaves simple, entire, elliptic to lanceolate, leathery, and dark green, and shiny above and often glaucous below; about 7–15 cm long and 2–3.5 cm wide, with many close inconspicuous parallel veins. The leaves taper to a more or less pointed tip, rounded at the base, with the margins curved down at the edge. Petioles short, from almost sessile up to 5 mm long. Cones egg-shaped at the end of the first year, about 5 cm long, and 3 cm in diameter, more or less round at the end of the second year, 8–10 cm in diameter. Female cones much larger than males, globular, on thick woody stalks, green, slightly glaucous, turning brownish during ripening. Seeds brown, small, ovoid to globose, flattened, winged, and attached to a triangular cone scale about 2.5 cm across. BIOLOGY Pacific kauri is monoecious and produces cones instead of flowers. The first female cones begin to be produced at about 10 years old and take up to 2 years to mature (more often in 12-15 months). -

Cupressaceae Et Taxodiaceae
AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de la Propriété Intellectuelle. articles L 335.2- L 335.10 http://www.cfcopies.com/V2/leg/leg_droi.php http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm ""&"$9 %%"'$%4$"'&%4$",,%&!($"!! !& "' !%&!""% >:<? +% #$,%!&,#"'$6"&!&"!'$ & !,"%!% #$ ' ! # # ##$ $&'$$$% 4!($%&,&$%"'$ "! /% %%%&!&#$"%%'$4!($%&,,%4""! $!$ &'+$ $"%%'$4!($%&, "$$! $!$" * -&$"!,$!%4!($%&, )"!8 ) "! % $,$$% 4!($%&, "$$! % !! '&( -&$"!,$!%4!($%&, "$$! ,"%%"'$% >:<?4!($%&, "$$!4 #'%!%5'&&% >79:?4<;<7=!0'($5+%5 !)4$! "E+$*4#-* PMNQ%))%+()A (#(")(*+())+)))+"%(*%( "*"$+G+L>(4C%))%+()H&+) '+)(%$%$#E,%(+""+)$ "E+$*4&%+((4")(***3)A (#(,,#$*#)+-(*+()*3).#%$ ")*$$ +*,"">'+ #E%$* &(#) (4")( *(," *3)A ) $%)) &&($$$*"+($$'+D E> *$)0-&(#(#)$3( (**+ &%+( "+( %$$> "+( )%+*$> "+( &*$ * "+( 4)$*4())#$* &%+( " *($)#))%$%$$))$$)$)+($*")'+*($$4)A (#(4"#$**%+)")##()+ +(.'+%$*&*4%$$("+(-&(*) &%+(4,"+(*(,"A (0"&(* %$! 7)!>#)(&&%(*+()$)'+J0 -

A Study in Ecological Economics
The Process of Forest Conservation in Vanuatu: A Study in Ecological Economics Luca Tacconi December 1995 A Thesis Submitted for the Degree of Doctor of Philosophy at The University of New South Wales I hereby declare that this submission is my own work and that, to the best of my . knowledge and belief, it contains no material previously published or written by another person nor material which to a substantial extent has been accepted for the award of any other degree or diploma of a university or other institute of higher ·learning, except where due acknowledgment is made in the text of the thesis. Luca Tacconi School of Economics and Management University College The University of New South Wales 22 December 1995 With love to my parents Alfi.o and Leda (Con affetto dedico questa tesi ai miei genitori Alfio e Leda) IV Abstract The objective of this thesis is to develop an ecological economic framework for the assessment and establishment of protected areas (PAs) that are aimed at conserving forests and biodiversity. The framework is intended to be both rigorous and relevant to the decision-making process. Constructivism is adopted as the paradigm guiding the research process of the thesis, after firstly examining also positivist philosophy and 'post-normal' scientific methodology. The tenets of both ecological and environmental economics are then discussed. An expanded model of human behaviour, which includes facets derived from institutional economics and socioeconomics as well as aspects of neoclassical economics, is outlined. The framework is further developed by considering, from a contractarian view point, the implications of intergenerational equity for biodiversity conservation policies. -

Syzygium and Related Genera (Myrtaceae) in Auckland
12 SYZYGIUM AND RELATED GENERA (MYRTACEAE) IN AUCKLAND R.O. Gardner The Australian members of this alliance have been expertly revised by Hyland (1983) making it possible to improve acquaintance with the five species in Syzygium Acmena and Waterhousea that are grown in Auckland for ornament and shelter. These are essentially trees of warm latitudes along Australias eastern coast but they find our climate and probably richer soils congenial and often it seems grow better here than in their homeland. None however have properly naturalized though undispersed juveniles and a few adults do occur. The disposition into genera is based largely upon characters of the. fruit and seed. What appears to be a simple baccate "monkey apple" may conceal unusual features like ruminate cotyledons or a missing seed coat so the fruits of these species produced here in abundance are very interesting to dissect and compare. Leaf silhouettes of the five Australian species are shown in Figure IA. Acmena smithii lillipilli monkey apple (NZ) Very common around Auckland as a street or specimen tree and in hedges. Seedlings occur close to the plantings but most succumb to scale and thrips. Some of the seedlings at Purewa cemetery have a lignotuber unlike most Australian forms of the species (Figure IB). Waterhousea floribunda weeping lillipilli (formerly Syzygium floribundum Eugenia ventenatii) Only seen in a few old gardens e.g. at Highwic The Pines Western Park being fine trees to c. 15 m tall 80 cm dbh and especially beautiful in spring with their pendent new foliage of pink and yellow; a species which should be much more often grown in this country. -

Biodiversity Summary: Port Phillip and Westernport, Victoria
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Republic of Fiji: the State of the World's Forest Genetic Resources
REPUBLIC OF FIJI This country report is prepared as a contribution to the FAO publication, The Report on the State of the World’s Forest Genetic Resources. The content and the structure are in accordance with the recommendations and guidelines given by FAO in the document Guidelines for Preparation of Country Reports for the State of the World’s Forest Genetic Resources (2010). These guidelines set out recommendations for the objective, scope and structure of the country reports. Countries were requested to consider the current state of knowledge of forest genetic diversity, including: Between and within species diversity List of priority species; their roles and values and importance List of threatened/endangered species Threats, opportunities and challenges for the conservation, use and development of forest genetic resources These reports were submitted to FAO as official government documents. The report is presented on www. fao.org/documents as supportive and contextual information to be used in conjunction with other documentation on world forest genetic resources. The content and the views expressed in this report are the responsibility of the entity submitting the report to FAO. FAO may not be held responsible for the use which may be made of the information contained in this report. STATE OF THE FOREST GENETIC RESOURCES IN FIJI Department of Forests Ministry of Fisheries and Forests for The Republic of Fiji Islands and the Secreatriat of Pacific Communities (SPC) State of the Forest Genetic Resources in Fiji _____________________________________________________________________________________________________________________ Table of Contents Executve Summary ………………………………………………………………………………………………………………………..…….. 5 Introduction ………………………………………………………………………………………………………………………………..…….. 6 Chapter 1: The Current State of the Forest Genetic Resources in Fiji ………………………………………………………………….……. -

Species Selection Guidelines Tree Species Selection
Species selection guidelines Tree species selection This section of the plan provides guidance around the selection of species for use as street trees in the Sunshine Coast Council area and includes region-wide street tree palettes for specific functions and settings. More specific guidance on signature and natural character palettes and lists of trees suitable for use in residential streets for each of the region's 27 Local plan areas are contained within Part B – Street tree strategies of the plan. Street tree palettes will be periodically reviewed as an outcome of street tree trials, the development of new species varieties and cultivars, or the advent of new pest or disease threats that may alter the performance and reliability of currently listed species. The plan is to be used in association with the Sunshine Coast Council Open Space Landscape Infrastructure Manual where guidance for tree stock selection (in line with AS 2303–2018 Tree stock for landscape use) and tree planting and maintenance specifications can be found. For standard advanced tree planting detail, maintenance specifications and guidelines for the selection of tree stock see also the Sunshine Coast Open Space Landscape Infrastructure Manual – Embellishments – Planting Landscape). The manual's Plant Index contains a comprehensive list of all plant species deemed suitable for cultivation in Sunshine Coast amenity landscapes. For specific species information including expected dimensions and preferred growing conditions see Palettes – Planting – Planting index). 94 Sunshine Coast Street Tree Master Plan 2018 Part A Tree nomenclature Strategic outcomes The names of trees in this document follow the • Trees are selected by suitably qualified and International code of botanical nomenclature experienced practitioners (2012) with genus and species given, followed • Tree selection is locally responsive and by the plant's common name. -

The Archaeology of Sulawesi Current Research on the Pleistocene to the Historic Period
terra australis 48 Terra Australis reports the results of archaeological and related research within the south and east of Asia, though mainly Australia, New Guinea and Island Melanesia — lands that remained terra australis incognita to generations of prehistorians. Its subject is the settlement of the diverse environments in this isolated quarter of the globe by peoples who have maintained their discrete and traditional ways of life into the recent recorded or remembered past and at times into the observable present. List of volumes in Terra Australis Volume 1: Burrill Lake and Currarong: Coastal Sites in Southern Volume 28: New Directions in Archaeological Science. New South Wales. R.J. Lampert (1971) A. Fairbairn, S. O’Connor and B. Marwick (2008) Volume 2: Ol Tumbuna: Archaeological Excavations in the Eastern Volume 29: Islands of Inquiry: Colonisation, Seafaring and the Central Highlands, Papua New Guinea. J.P. White (1972) Archaeology of Maritime Landscapes. G. Clark, F. Leach Volume 3: New Guinea Stone Age Trade: The Geography and and S. O’Connor (2008) Ecology of Traffic in the Interior. I. Hughes (1977) Volume 30: Archaeological Science Under a Microscope: Studies in Volume 4: Recent Prehistory in Southeast Papua. B. Egloff (1979) Residue and Ancient DNA Analysis in Honour of Thomas H. Loy. M. Haslam, G. Robertson, A. Crowther, S. Nugent Volume 5: The Great Kartan Mystery. R. Lampert (1981) and L. Kirkwood (2009) Volume 6: Early Man in North Queensland: Art and Archaeology Volume 31: The Early Prehistory of Fiji. G. Clark and in the Laura Area. A. Rosenfeld, D. Horton and J. Winter A. -

Myrtle Rust Reviewed the Impacts of the Invasive Plant Pathogen Austropuccinia Psidii on the Australian Environment R
Myrtle Rust reviewed The impacts of the invasive plant pathogen Austropuccinia psidii on the Australian environment R. O. Makinson 2018 DRAFT CRCPLANTbiosecurity CRCPLANTbiosecurity © Plant Biosecurity Cooperative Research Centre, 2018 ‘Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment’ is licenced by the Plant Biosecurity Cooperative Research Centre for use under a Creative Commons Attribution 4.0 Australia licence. For licence conditions see: https://creativecommons.org/licenses/by/4.0/ This Review provides background for the public consultation document ‘Myrtle Rust in Australia – a draft Action Plan’ available at www.apbsf.org.au Author contact details R.O. Makinson1,2 [email protected] 1Bob Makinson Consulting ABN 67 656 298 911 2The Australian Network for Plant Conservation Inc. Cite this publication as: Makinson RO (2018) Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment. Plant Biosecurity Cooperative Research Centre, Canberra. Front cover: Top: Spotted Gum (Corymbia maculata) infected with Myrtle Rust in glasshouse screening program, Geoff Pegg. Bottom: Melaleuca quinquenervia infected with Myrtle Rust, north-east NSW, Peter Entwistle This project was jointly funded through the Plant Biosecurity Cooperative Research Centre and the Australian Government’s National Environmental Science Program. The Plant Biosecurity CRC is established and supported under the Australian Government Cooperative Research Centres Program. EXECUTIVE SUMMARY This review of the environmental impacts of Myrtle Rust in Australia is accompanied by an adjunct document, Myrtle Rust in Australia – a draft Action Plan. The Action Plan was developed in 2018 in consultation with experts, stakeholders and the public. The intent of the draft Action Plan is to provide a guiding framework for a specifically environmental dimension to Australia’s response to Myrtle Rust – that is, the conservation of native biodiversity at risk. -

Summary Report on Forests of the Mataqali Nadicake Kilaka, Kubulau District, Bua, Vanua Levu
SUMMARY REPORT ON FORESTS OF THE MATAQALI NADICAKE KILAKA, KUBULAU DISTRICT, BUA, VANUA LEVU By Gunnar Keppel (Biology Department, University of the South Pacific) INTRODUCTION I was approached by Dr. David Olson of the Wildlife Conservation Society (WCS) to assess the type, status and quality of the forest in Kubulau District, Bua, Vanua Levu. I initially spent 2 days, Friday (28/10/2005) afternoon and the whole of Saturday (29/10/2005), in Kubulau district. This invitation was the result of interest by some landowning family clans (mataqali) to protect part of their land and the offer by WCS to assist in reserving part of their land for conservation purposes. On Friday I visited two forest patches (one logged about 40 years ago and another old-growth) near the coast and Saturday walking through the forests in the center of the district. Because of the scarcity of data obtained (and because the forest appeared suitable for my PhD research), I decided to return to the district for a more detailed survey of the northernmost forests of Kubulau district from Saturday (12/11/2005) to Tuesday (22/11/2005). Upon returning, I found out that the mataqali Nadicake Nadi had abandoned plans to set up a reserve and initiated steps to log their forests. Therefore, I decided to focus my research on the land of the mataqali Nadicake Kilaka only. My objectives were the following: 1) to determine the types of vegetation present 2) to produce a checklist of the flora and, through this list, identify rare and threatened species in the reserve 3) to undertake a quantitative survey of the northernmost forests (lowland tropical rain forest) by setting up 4 permanent 50 ×50m plots 4) to assess the status of the forests 5) to determine the state and suitability of the proposed reserve 6) to assess possible threats to the proposed reserve. -
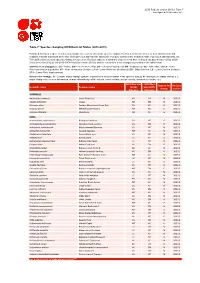
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying