Kraehenmann GA-MS CN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Relaxation Techniques? a Substantial Amount of Research Has Been Done on Relaxation Techniques
U.S. Department of Health & Human Services National Institutes of Health Relaxation Techniques © Thinkstock What’s the Bottom Line? How much do we know about relaxation techniques? A substantial amount of research has been done on relaxation techniques. However, for many health conditions, the number or size of the studies has been small, and some studies have been of poor quality. What do we know about the effectiveness of relaxation techniques? Relaxation techniques may be helpful in managing a variety of health conditions, including anxiety associated with illnesses or medical procedures, insomnia, labor pain, chemotherapy-induced nausea, and temporomandibular joint dysfunction. Psychological therapies, which may include relaxation techniques, can help manage chronic headaches and other types of chronic pain in children and adolescents. Relaxation techniques have also been studied for other conditions, but either they haven’t been shown to be useful, research results have been inconsistent, or the evidence is limited. What do we know about the safety of relaxation techniques? Relaxation techniques are generally considered safe for healthy people, although there have been a few reports of negative experiences, such as increased anxiety. People with serious physical or mental health problems should discuss relaxation techniques with their health care providers. What Are Relaxation Techniques? Relaxation techniques include a number of practices such as progressive relaxation, guided imagery, biofeedback, self-hypnosis, and deep breathing exercises. The goal is similar in all: to produce the body’s natural relaxation response, characterized by slower breathing, lower blood pressure, and a feeling of increased well-being. Meditation and practices that include meditation with movement, such as yoga and tai chi, can also promote relaxation. -

Trauma-Focused Group Music and Imagery with Women Suffering From
Approaches: An Interdisciplinary Journal of Music Therapy | Special Issue 9 (2) 2017 SPECIAL ISSUE Guided Imagery and Music: Contemporary European perspectives and developments Article Trauma-focused group music and imagery with women suffering from PTSD/complex PTSD: A feasibility study Gabriella Rudstam, Ulf Elofsson, Hans Peter Søndergaard, Lars Ole Bonde & Bolette Daniels Beck ABSTRACT Women who have been exposed to physical, psychological and/or sexual abuse, often with a history of childhood abuse and neglect, frequently suffer from post-traumatic stress disorder (PTSD) or complex post- traumatic stress disorder (CPTSD). However, the evidence-based treatments recommended for this population help only 50%, so there is a need to investigate complementary methods. In this study one such promising method has been explored: trauma-focused Group Music and Imagery (GrpMI). In a non- randomised clinical setting the feasibility of GrpMI and the suitability of chosen measurements were explored. Ten participants with PTSD/CPTSD were enrolled in the pilot study, five in each group. All participants completed the treatment. The primary outcome was symptoms of PTSD measured at pre-, post- and follow-up. The secondary outcomes were dissociation and quality of life. The results showed a decrease in PTSD and dissociative symptoms, and an increase in quality of life following treatment. This tendency was maintained at follow-up. An analysis of individual, semi-structured interviews with the participants after the termination of the treatment showed that the participants found the group treatment helpful and acceptable. Since the findings indicate that trauma-focused GrpMI has a positive effect on the psychological health of the women, a larger randomised controlled study is needed. -

Healing Factors in Guided Affective Imagery: a Qualitative Meta-Analysis
Healing Factors in Guided Affective Imagery: A Qualitative Meta-Analysis Submitted in Partial Fulfillment Of the Requirements for the Degree of Doctor of Philosophy with a concentration in Psychology and a specialization in Counseling and Psychotherapy at the Union Institute & University Cincinnati, Ohio Elaine Sue Kramer April 3, 2010 Core Faculty: Lawrence J. Ryan, PhD i Abstract This qualitative meta-analysis compares and contrasts European and American approaches to Guided Affective Imagery (GAI). From a comparative review of literature of the European and American approaches, it is observed that there are noteworthy differences in how GAI is understood in theory and applied in practice. In the United States, GAI is not perceived as a method of deep psychotherapeutic intervention for neurotic disorders by most practitioners. In Europe, GAI is one of the more prevalent intervention techniques that has been reported to be effective in many disorders. Secondly, this meta-analysis seeks to identify the essential healing elements of GAI as they are implemented in psychotherapy. Twelve factors are identified from the literature. Of fundamental clinical importance is the activation of a patient’s “resources,” the positive characteristics of an individual that can be accessed to reinforce the patient’s ability to deal with a past traumatic experience. GAI provides the imagery context by which the patient may re-experience the trauma. The therapist assists by encouraging the patient to repeatedly utilize internal resources to confront the fearful event. Lasting relief may be conceptualized as repeated resource activation leading to a biochemically-induced remapping at synaptic sites away from the limbic-centered, emotion-based neural path associated with the traumatic event toward the prefrontal cortex-centered, cognitive-based path of appropriate behavior. -

List of Psycho Therapy Spirits for MD 12 Steps Programs, 100 Years Of
List of Psycho Therapy Spirits for MD 12 steps programs, 100 Years of Psychotherapy – And the World's Getting Worse, abnormal Psychotherapy, Abreaction, Academy at Dundee Ranch, Academy at Ivy Ridge, Academy at Swift River, Academy of Cognitive Therapy, Accelerated experiential dynamic therapy, Acceptance and commitment therapy, Ackerman Institute for the Family, Active listening, Activity theory, Adaptive psychotherapy, Addiction psychiatry, Addictions Anonymous, Adlerian therapy, Adventure therapy, Affect logic, Affect theory, Afterburn, Aggression Replacement Training, Alcoholics Anonymous, altered emotions, altered mind, altered soul, altered state of consciousness, altered will, Alternative new age therapies, Alternative therapies for developmental and learning disabilities, alters, Amplification, Analytical psychology, Anger management, Animal-assisted therapy, Anomalistic psychology, anti-christ, Anti-psychiatry, Anti-psychology, Anxiety Management Training, anxiety reduction technique, Anything Anonymous, Apex effect, Applied Behavioral Analysis, Applied Psychophysiology and Biofeedback, Arbitrary inference, Art therapy, Asian psychology, Aspen Achievement Academy, Assertive community treatment, Atavistic regression, Attachment in adults, Attachment in children, Attachment measures, Attachment theory, Attachment therapy, Attachment-based psychotherapy, Attachment-based therapy for children, Attack therapy, Audio–visual entrainment, Auditing, Autogenic training, Autosuggestion, Auxiliary ego, Aversion therapy, Aylan School, Bad -

Post Traumatic Stress Disorder and the Benefits of Guided Mental Imagery in Treatment
University of Northern Iowa UNI ScholarWorks Graduate Research Papers Student Work 2006 Post traumatic stress disorder and the benefits of guided mental imagery in treatment Lisa A. Langstraat University of Northern Iowa Let us know how access to this document benefits ouy Copyright ©2006 Lisa A. Langstraat Follow this and additional works at: https://scholarworks.uni.edu/grp Part of the Counseling Commons, Education Commons, and the Mental Disorders Commons Recommended Citation Langstraat, Lisa A., "Post traumatic stress disorder and the benefits of guided mental imagery in treatment" (2006). Graduate Research Papers. 1077. https://scholarworks.uni.edu/grp/1077 This Open Access Graduate Research Paper is brought to you for free and open access by the Student Work at UNI ScholarWorks. It has been accepted for inclusion in Graduate Research Papers by an authorized administrator of UNI ScholarWorks. For more information, please contact [email protected]. Post traumatic stress disorder and the benefits of guided mental imagery in treatment Abstract Seventy percent of adults in the United States will experience at least one traumatic event in their lifetime. Out of these individuals, 25% will develop post-traumatic stress disorder (PTSD). This disorder is characterized by distinct physiological changes as well as notable psychological symptoms. If left untreated or improperly treated, PTSD exacts significant costs in individual suffering, quality of life, interpersonal relationships, productivity, and increased use of medical and psychiatric services. The purpose of this paper is to present comprehensive information about PTSD and its impact on those who suffer from the disorder. This paper will also provide a brief synopsis of traditional treatment, and explore the benefits of utilizing guided mental imagery as an essential component of effective treatment. -

The Effectiveness of Guided Imagery in Treating Compassion Fatigue
The Effectiveness of Guided Imagery in Treating Compassion Fatigue and Anxiety of Mental Health Workers Downloaded from https://academic.oup.com/swr/article-abstract/42/1/33/4788592 by University of Texas at Austin user on 29 July 2019 Kimberly A. Kiley, Ashwini R. Sehgal, Susan Neth, Jacqueline Dolata, Earl Pike, James C. Spilsbury, and Jeffrey M. Albert Mental health professionals’ exposure to clients’ traumatic experiences can result in elevated stress, including compassion fatigue and burnout. Experiencing symptoms of these types of stress can hinder workers’ ability to provide effective services. If a tool can reduce these symp- toms, there is potential benefit for workers as well as those receiving their services. The purpose of this study was to examine the effects of prerecorded guided imagery (GI) on compassion fatigue and state anxiety. A total of 69 employees of a mental health nonprofit organization participated in this two-arm randomized controlled trial. Participants completed the Profes- sional Quality of Life Scale, the Perceived Stress Scale, and question 6 from the Pittsburgh Sleep Quality Index at baseline and follow-up, and completed State Trait Anxiety Inventory short form before and after each activity (GI or taking a break). Results revealed statistically sig- nificant differences in change scores between the control and experimental groups for state anxiety and sleep quality. The results suggest that GI may be useful for reducing stress for men- tal health professionals, which could have positive implications for quality of service delivery. KEY WORDS: compassion fatigue; guided imagery; mental health workers; self-care; sleep growing body of literature suggests that from which ProQOL is derived, helping profes- exposure to clients’ traumatic experiences can sionals’ quality of life has both positive and negative A have negative effects on helping professionals aspects. -

The Nightmare Free
FREE THE NIGHTMARE PDF lars Kepler | 608 pages | 03 Jan 2013 | HarperCollins Publishers | 9780007414505 | English | London, United Kingdom The Nightmare - Wikipedia The Nightmare By Henry Fuseli. Regarded as one of the The Nightmare Paintings Ever. For the meaning of other celebrated masterpieces, please see: Famous Paintings Analyzed One of the most innovative Romantic artists of his day, the Swiss-born Johann Heinrich Henry Fuseli - son of the The Nightmare Johann Caspar Fussli - developed an early talent for drawing before moving to London The Nightmare at the age of Here, encouraged by Joshua Reynolds who was shortly to be elected the first president of the newly formed Royal Academy of ArtsFuseli took up painting. This led him to spend most of the s in Italy, studying the figure painting of Michelangelo which became a major influence on his art. Other influences included 16th-century Mannerism and literary sources, notably Shakespeare. Later appointed a professor of painting at the Royal Academy, he became one of the best English painters of the eighteenth century and was buried in St Paul's Cathedral. Like his The Nightmare contemporary William BlakeFuseli's strength as a painter lies in his imaginative intensity, and The Nightmare - which he sold for 20 guineas - remains his greatest and most baffling masterpiece. Overlooked after his death, Fuseli was 'rediscovered' by 20th-century Expressionists and Surrealists who greatly admired his creativity. Painted shortly after his return from Italy, The Nightmare was first shown to the public in The Nightmare the annual exhibition of the Royal Academy. An instant success, it established Fuseli's reputation as one of the most creative artists in London. -

A Qualitative Study on the Use of Guided Imagery and Music, Expressive Arts, and a Body-Centered Perspective to Address Women’S Issues
BREAKING THE SILENCE: A QUALITATIVE STUDY ON THE USE OF GUIDED IMAGERY AND MUSIC, EXPRESSIVE ARTS, AND A BODY-CENTERED PERSPECTIVE TO ADDRESS WOMEN’S ISSUES A Thesis by CYNTHIA TATE, MT-BC Submitted to the Graduate School Appalachian State University in partial fulfillment of the requirements for the degree of MASTER OF MUSIC THERAPY August 2014 Hayes School of Music BREAKING THE SILENCE: A QUALITATIVE STUDY ON THE USE OF GUIDED IMAGERY AND MUSIC, EXPRESSIVE ARTS, AND A BODY-CENTERED PERSPECTIVE TO ADDRESS WOMEN’S ISSUES A Thesis by CYNTHIA TATE August 2014 APPROVED BY: Cathy H. McKinney Chairperson, Thesis Committee Christine P. Leist Member, Thesis Committee Marianne Adams Member, Thesis Committee William L. Pelto Dean, Hayes School of Music Max C. Poole, Ph.D. Dean, Cratis Williams Graduate School Copyright by Cynthia Tate 2014 All Rights Reserved Abstract BREAKING THE SILENCE: A QUALITATIVE STUDY ON THE USE OF GUIDED IMAGERY AND MUSIC, EXPRESSIVE ARTS, AND A BODY-CENTERED PERSPECTIVE TO ADDRESS WOMEN’S ISSUES Cynthia Tate Music Therapist – Board Certified B.S., North Carolina State University B.M., Appalachian State University M.M.T., Appalachian State University Chairperson: Cathy H. McKinney Issues related to the body-mind connection are endemic in our society. Due to gender-specific factors, women can be at a greater risk for disorders or characteristics that result from an unhealthy relationship to the body. The Bonny Method of Guided Imagery and Music (GIM) has been used to address somatic issues such as those that manifest in trauma and illness and has the potential to create powerful changes in the body and mind. -

Guided Imagery Therapy
IOSR Journal of Nursing and Health Science (IOSR-JNHS) e-ISSN: 2320–1959.p- ISSN: 2320–1940 Volume 4, Issue 5 Ver. III (Sep. - Oct. 2015), PP 56-58 www.iosrjournals.org Guided Imagery Therapy P. Kodeeswara Prabu, Dr. Jeyagowri Subhash, 1Ph.D., Scholar, Rani Meyyammai College of Nursing, Chidambaram, Tamilnadu. 2Vice-Principal, Rani Meyyammai College of Nursing, Chidambaram, Tamilnadu. A mental image can be defined as “a thought with sensory qualities.” It is something we mentally see, hear, taste, smell, touch, or feel. The term “guided imagery” refers to a wide variety of techniques, including simple visualization and direct suggestion using imagery, metaphor and story-telling, fantasy exploration and game playing, dream interpretation, drawing, and active imagination where elements of the unconscious are invited to appear as images that can communicate with the conscious mind. Once considered an “alternative” “or complementary” approach, guided imagery is now finding widespread scientific and public acceptance, and it is being used to teach psycho physiological relaxation, alleviate anxiety and depression, relieve physical and psychological symptoms, overcome health-endangering habits, resolve conflicts, and help patients prepare for surgery and tolerate procedures more comfortably. Mental images, formed long before we learn to understand and use words, lie at the core of who we think we are, what we believe the world is like, what we feel we deserve, what we think will happen to us, and how motivated we are to take care of ourselves. These images strongly influence our beliefs and attitudes about how we fall ill, and what will help us to get better. -

CARE with Guided Imagery
10/31/2016 C.A.R.E. with Guided Imagery: How to Expand the Therapeutic Benefits of C.A.R.E. Programming Susan E. Mazer, Ph.D. President & CEO Welcome & Agenda • Context for the discussion • What is Guided Imagery? • Benefits of Guided Imagery • C.A.R.E. with Guided Imagery The identification and management of pain is an important component of [patient]‐centered care. [Patients] can expect that their health care providers will involve them in their assessment and management of pain. Both pharmacologic and nonpharmacologic strategies have a role in the management of pain. 1 10/31/2016 Acupuncture Chiropractic therapy Osteopathic manipulative treatment Massage therapy Physical therapy Relaxation therapy Cognitive behavioral therapy Acupuncture Chiropractic therapy Osteopathic manipulative treatment Massage therapy Physical therapy Relaxation therapy > guided imagery Cognitive behavioral therapy Melzack/Wall: Gate‐Control Theory Neurological, psychological, physiological. Non‐painful stimuli can lessen perception of pain. 2 10/31/2016 Melzack/Wall: Gate‐Control Theory Distraction theory: Focusing on non‐painful stimuli can lessen perception of pain. Thoughts, anxiety about pain will increase perception of pain. Pharmacological and non‐pharmacological means for patient management are optimal. Suffering is silent. GI Whole Person Care 3 10/31/2016 Whole Person Care “A body does not suffer, only a person does…making decisions based primarily on the sick person rather than the disease, maximizing function rather than merely length of life, and -

June 8, 2014 Berkeley, California, USA
I J o D R Abstracts of the 31th Annual Conference of the International Association for the Study of Dreams June 4 - June 8, 2014 Berkeley, California, USA Content This supplement of the International Journal of Dream Research includes the abstracts of presenters who gave consent to the publishing. The abstracts are categorized into thematic groups and within the category sorted according to the last name of the fi rst presenter. Affi liations are included only for the fi rst author. A name register at the end is also provided. ing on their own behalf and asking for response. The Global Dream Initiative will develop a forum to see and hear the world’s dreams and to begin utilizing them to create new and more generative ways of responding to the trauma of the world, ways that are not trapped in the cultural, politi- cal, economic, and environmental approaches that now are failing us. Joining other like-minded efforts worldwide, the Contents: Global Dream Initiative is a call to action. 1. Keynotes 2. Morning Dream Groups Sleep and Dreams as Pathways to Resilience Fol- 3. Workshops lowing Trauma 4. Clinical Topics Anne Germain 5. Religion/Spiritual/Culture/Arts Pittsburgh, PA, USA 6. Education/Other Topics 7. PSI Dreaming Sleep is a fundamental brain function, and a core biological process involved in sustaining mental and physical readi- 8. Lucid Dreaming ness, especially when facing adversity. Sleep is essential for 9. Research/Theory survival and is involved in a number of biological and men- tal functions that sustain performance, including emotion 10. -
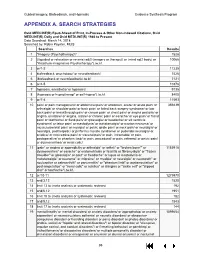
Guided Imagery, Biofeedback, and Hypnosis: a Map of the Evidence
Guided Imagery, Biofeedback, and Hypnosis Evidence Synthesis Program APPENDIX A. SEARCH STRATEGIES Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Date Searched: March 14, 2018 Searched by: Robin Paynter, MLIS Searches Results 1 "Imagery (Psychotherapy)"/ 1528 2 (((guided or relaxation or reverie) adj3 (imagery or therapy)) or (mind adj2 body) or 10066 "Katathym-imaginative Psychotherapy").tw,kf. 3 or/1-2 11235 4 biofeedback, psychology/ or neurofeedback/ 7426 5 (biofeedback or neurofeedback).tw,kf. 7121 6 or/4-5 10378 7 hypnosis, anesthetic/ or hypnosis/ 9135 8 (hypnosis or hypnotherap* or self-hypno*).tw,kf. 8400 9 or/7-8 11593 10 pain/ or pain management/ or abdominal pain/ or abdomen, acute/ or acute pain/ or 359439 arthralgia/ or shoulder pain/ or back pain/ or failed back surgery syndrome/ or low back pain/ or breakthrough pain/ or cancer pain/ or chest pain/ or angina pectoris/ or angina, unstable/ or angina, stable/ or chronic pain/ or earache/ or eye pain/ or facial pain/ or toothache/ or flank pain/ or glossalgia/ or headache/ or slit ventricle syndrome/ or labor pain/ or mastodynia/ or metatarsalgia/ or morton neuroma/ or musculoskeletal pain/ or myalgia/ or pelvic girdle pain/ or neck pain/ or neuralgia/ or neuralgia, postherpetic/ or piriformis muscle syndrome/ or pudendal neuralgia/ or sciatica/ or nociceptive pain/ or visceral pain/ or pain, intractable/ or pain, postoperative/ or phantom limb/ or pain, procedural/ or pain, referred/