Anthrax in Humans and Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MS Hatch-Waxman PTE REQUEST for EXTENSION of PATENT
Docket No.: 029420.0155-US02 (PATENT) IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In re United States Patent of: Alan J. Korman, et aL. Patent No.: 7,605,238 Issued: October 20, 2009 For: HUMAN CTLA-4 ANTIBODIES MS Hatch-Waxman PTE Commissioner for Patents Offce of Patent Legal Administration Room MDW 7D55 600 Dulany Street (Madison Building) Alexandria, VA 223 14 REQUEST FOR EXTENSION OF PATENT TERM UNDER 35 U.S.C. §156 Sir: Pursuant to 35 U.S.C. §156 and 37 C.F.R. §§1.710-1.791, Medarex, Inc., the current address of which is Route 206 and Province Line Road, Princeton, New Jersey 08540 ("Applicant" or "Medarex"), hereby requests an extension of U.S. Patent No. 7,605,238 (the "'238 patent"). As permitted by 37 C.F.R. §1.785(b) and MPEP §2761, Applicant is concurrently fiing a request for patent term extension of U.S. Patent No. 6,984,720 based upon the same regulatory review period. Medarex represents that it is the owner and assignee of the entire interest in and to Letters Patent of the United States No. 7,605,238 (Exhibit 1) granted to Alan J. Korman, Edward L. Halk, Nils Lonberg, Yashwant M. Deo and Tibor P. Keler (the "inventors") on October 20, 2009, for "Human CTLA-4 Antibodies and Their Uses" by virtue of an assignment from the DC; 3983891.1 Patent No.: 7,605,238 - 2- Docket No.: 029420.0155-US02 Alan J. Korman, Edward L. Halk and Nils Lonberg to Medarex, recorded in the United States Patent and Trademark Offce ("PTO") on March 6, 2003 at Reel 013817, Frame 0628 and an assignment from Yashwant M. -

B2bi 2.5 Support Reference Catalog 3 Accessibility
B2Bi Version 2.5 April 2019 Support Reference Catalog Copyright © 2019 Axway All rights reserved. This documentation describes the following Axway software: Axway B2Bi 2.5 No part of this publication may be reproduced, transmitted, stored in a retrieval system, or translated into any human or computer language, in any form or by any means, electronic, mechanical, magnetic, optical, chemical, manual, or otherwise, without the prior written permission of the copyright owner, Axway. This document, provided for informational purposes only, may be subject to significant modification. The descriptions and information in this document may not necessarily accurately represent or reflect the current or planned functions of this product. Axway may change this publication, the product described herein, or both. These changes will be incorporated in new versions of this document. Axway does not warrant that this document is error free. Axway recognizes the rights of the holders of all trademarks used in its publications. The documentation may provide hyperlinks to third-party web sites or access to third-party content. Links and access to these sites are provided for your convenience only. Axway does not control, endorse or guarantee content found in such sites. Axway is not responsible for any content, associated links, resources or services associated with a third-party site. Axway shall not be liable for any loss or damage of any sort associated with your use of third-party content. Contents Accessibility 4 Accessibility features of B2Bi 4 Keyboard -

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

Investigation of Anthrax Associated with Intentional Exposure
October 19, 2001 / Vol. 50 / No. 41 889 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 893 Recognition of Illness Associated with the Intentional Release of a Biologic Agent 897 Weekly Update: West Nile Virus Activity — United States, October 10–16, 2001 Update: Investigation of Anthrax Associated with Intentional Exposure and Interim Public Health Guidelines, October 2001 On October 4, 2001, CDC and state and local public health authorities reported a case of inhalational anthrax in Florida (1 ). Additional cases of anthrax subsequently have been reported from Florida and New York City. This report updates the findings of these case investigations, which indicate that infections were caused by the intentional release of Bacillus anthracis. This report also includes interim guidelines for postexposure pro- phylaxis for prevention of inhalational anthrax and other information to assist epidemi- ologists, clinicians, and laboratorians responding to intentional anthrax exposures. For these investigations, a confirmed case of anthrax was defined as 1) a clinically compatible case of cutaneous, inhalational, or gastrointestinal illness* that is laboratory confirmed by isolation of B. anthracis from an affected tissue or site or 2) other labora- tory evidence of B. anthracis infection based on at least two supportive laboratory tests. A suspected case was defined as 1) a clinically compatible case of illness without isola- tion of B. anthracis and no alternative diagnosis, but with laboratory evidence of B. anthracis by one supportive laboratory test or 2) a clinically compatible case of an- thrax epidemiologically linked to a confirmed environmental exposure, but without cor- roborative laboratory evidence of B. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

The First but Hopefully Not the Last: How the Last of Us Redefines the Survival Horror Video Game Genre
The College of Wooster Open Works Senior Independent Study Theses 2018 The First But Hopefully Not the Last: How The Last Of Us Redefines the Survival Horror Video Game Genre Joseph T. Gonzales The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Arts and Humanities Commons, and the Other Film and Media Studies Commons Recommended Citation Gonzales, Joseph T., "The First But Hopefully Not the Last: How The Last Of Us Redefines the Survival Horror Video Game Genre" (2018). Senior Independent Study Theses. Paper 8219. This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The College of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2018 Joseph T. Gonzales THE FIRST BUT HOPEFULLY NOT THE LAST: HOW THE LAST OF US REDEFINES THE SURVIVAL HORROR VIDEO GAME GENRE by Joseph Gonzales An Independent Study Thesis Presented in Partial Fulfillment of the Course Requirements for Senior Independent Study: The Department of Communication March 7, 2018 Advisor: Dr. Ahmet Atay ABSTRACT For this study, I applied generic criticism, which looks at how a text subverts and adheres to patterns and formats in its respective genre, to analyze how The Last of Us redefined the survival horror video game genre through its narrative. Although some tropes are present in the game and are necessary to stay tonally consistent to the genre, I argued that much of the focus of the game is shifted from the typical situational horror of the monsters and violence to the overall narrative, effective dialogue, strategic use of cinematic elements, and character development throughout the course of the game. -

The File Cmfonts.Fdd for Use with Latex2ε
The file cmfonts.fdd for use with LATEX 2".∗ Frank Mittelbach Rainer Sch¨opf 2019/12/16 This file is maintained byA theLTEX Project team. Bug reports can be opened (category latex) at https://latex-project.org/bugs.html. 1 Introduction This file contains the external font information needed to load the Computer Modern fonts designed by Don Knuth and distributed with TEX. From this file all .fd files (font definition files) for the Computer Modern fonts, both with old encoding (OT1) and Cork encoding (T1) are generated. The Cork encoded fonts are known under the name ec fonts. 2 Customization If you plan to install the AMS font package or if you have it already installed, please note that within this package there are additional sizes of the Computer Modern symbol and math italic fonts. With the release of LATEX 2", these AMS `extracm' fonts have been included in the LATEX font set. Therefore, the math .fd files produced here assume the presence of these AMS extensions. For text fonts in T1 encoding, the directive new selects the new (version 1.2) DC fonts. For the text fonts in OT1 and U encoding, the optional docstrip directive ori selects a conservatively generated set of font definition files, which means that only the basic font sizes coming with an old LATEX 2.09 installation are included into the \DeclareFontShape commands. However, on many installations, people have added missing sizes by scaling up or down available Metafont sources. For example, the Computer Modern Roman italic font cmti is only available in the sizes 7, 8, 9, and 10pt. -

Bacillus Cereus Pneumonia
‘Advances in Medicine and Biology’, Volume 67, 2013 Nova Science Publisher Inc., Editor: Berhardt LV To Alessia, my queen, and Giorgia, my princess Chapter BACILLUS CEREUS PNEUMONIA Vincenzo Savini* Clinical Microbiology and Virology, Spirito Santo Hospital, Pescara (PE), Italy ABSTRACT Bacillus cereus is a Gram positive/Gram variable environmental rod, that is emerging as a respiratory pathogen; particularly, pneumonia it causes may be serious and can resemble the anthrax disease. In fact, the organism is strictly related to the famous Bacillus anthracis, with which it shares genotypical, fenotypical, and pathogenic features. Treatment of B. cereus lower airway infections is becoming increasingly hard, due to the spread of multidrug resistance traits among members of the species. Hence, the present chapter’s scope is to shed a light on this bacterium’s lung pathogenicity, by depicting salient microbiological, epidemiological and clinical features it shows. Also, we would like to explore virulence determinants and resistance mechanisms that make B. cereus a life-threatening, potentially difficult-to-treat agent of airway pathologies. * Email: [email protected]. 2 Vincenzo Savini INTRODUCTION The genus Bacillus includes spore-forming species, strains of which are not usually considered to be clinically relevant when isolated from human specimens; in fact, these bacteria are known to be ubiquitously distributed in the environment and may easily contaminate culture material along with improperly handled sample collection devices (Miller, 2012; Brooks, 2001). Bacillus anthracis is the prominent agent of human pathologies within the genus Bacillus, although it is uncommon in most clinical laboratories (Miller, 2012). It is a frank pathogen, as it causes skin and enteric infections; above all, however, it is responsible for a serious lung disease (the ‘anthrax’) that is frequently associated with bloodstream infection and a high mortality rate (Frankard, 2004). -

Oracle® Database Installation Guide
Oracle® Database Installation Guide 18c for Microsoft Windows E83888-01 August 2018 Oracle Database Installation Guide, 18c for Microsoft Windows E83888-01 Copyright © 1996, 2018, Oracle and/or its affiliates. All rights reserved. Primary Authors: Tanaya Bhattacharjee, Sunil Surabhi Contributing Authors: Prakash Jashnani, Mark Bauer, Bharathi Jayathirtha Contributors: Barb Glover, Eric Belden, Sudip Datta, David Friedman, Alex Keh, Peter LaQuerre, Rich Long, Matt McKerley, Sham Rao Pavan, Hanlin Qian, Sujatha Tolstoy, Sergiusz Wolicki, Sue Mavris, Mohammed Shahnawaz Quadri, Vishal Saxena, Krishna Itikarlapall, , Santanu Datta, Christian Shay, Aneesh Khanderwal, Michael Coulter, Robert Achacoso, Malai Stalin, David Price, Ramesh Chakravarthula This software and related documentation are provided under a license agreement containing restrictions on use and disclosure and are protected by intellectual property laws. Except as expressly permitted in your license agreement or allowed by law, you may not use, copy, reproduce, translate, broadcast, modify, license, transmit, distribute, exhibit, perform, publish, or display any part, in any form, or by any means. Reverse engineering, disassembly, or decompilation of this software, unless required by law for interoperability, is prohibited. The information contained herein is subject to change without notice and is not warranted to be error-free. If you find any errors, please report them to us in writing. If this is software or related documentation that is delivered to the U.S. Government or anyone licensing it on behalf of the U.S. Government, then the following notice is applicable: U.S. GOVERNMENT END USERS: Oracle programs, including any operating system, integrated software, any programs installed on the hardware, and/or documentation, delivered to U.S. -

Sound, Embodiment, and the Experience of Interactivity in Video Games & Virtual
Sound, Embodiment, and the Experience of Interactivity in Video Games & Virtual Environments –DRAFT—citations incomplete! Lori Landay, Professor of Cultural Studies Berklee College of Music This presentation is part of a larger project that explores perception and imagination in interactive media, and how they are created by sound, image, and action. It marks an early stage in extending my previous work on the virtual kinoeye in virtual worlds and video games to consider aural, haptic, and kinetic aspects of embodiment and interactivity. SLIDE 2 2 In particular, the argument is that innovative sound has the potential to bridge the gaps in experiencing embodiment caused by disconnections between the perceived body in physical, representational, and imagined contexts. To explore this, I draw on Walter Murch’s spectrum of encoded and embodied sound, interviews with sound designers and composers, ways of thinking about the body from phenomenology, video game studies scholarship, and examples of the relationship between sound effects and music in video games. Let’s start with a question philosopher Don Ihde poses when he asks his students to imagine then describe an activity they have never done. Often students choose jumping out of an airplane with a parachute, and as he works through the distinctions between the possible senses of one’s own body as the physical body, a first-person perspective of the perceiving body, which 3 he calls the here-body, or the objectified over-there-body, the third-person view of one’s own body, he asks, “Where does one feel the wind?” Ihde argues the full multidimensional sensory experience is in the embodied perspective. -
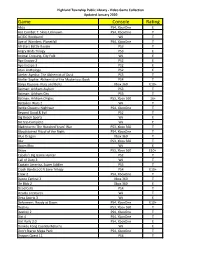
Game Console Rating
Highland Township Public Library - Video Game Collection Updated January 2020 Game Console Rating Abzu PS4, XboxOne E Ace Combat 7: Skies Unknown PS4, XboxOne T AC/DC Rockband Wii T Age of Wonders: Planetfall PS4, XboxOne T All-Stars Battle Royale PS3 T Angry Birds Trilogy PS3 E Animal Crossing, City Folk Wii E Ape Escape 2 PS2 E Ape Escape 3 PS2 E Atari Anthology PS2 E Atelier Ayesha: The Alchemist of Dusk PS3 T Atelier Sophie: Alchemist of the Mysterious Book PS4 T Banjo Kazooie- Nuts and Bolts Xbox 360 E10+ Batman: Arkham Asylum PS3 T Batman: Arkham City PS3 T Batman: Arkham Origins PS3, Xbox 360 16+ Battalion Wars 2 Wii T Battle Chasers: Nightwar PS4, XboxOne T Beyond Good & Evil PS2 T Big Beach Sports Wii E Bit Trip Complete Wii E Bladestorm: The Hundred Years' War PS3, Xbox 360 T Bloodstained Ritual of the Night PS4, XboxOne T Blue Dragon Xbox 360 T Blur PS3, Xbox 360 T Boom Blox Wii E Brave PS3, Xbox 360 E10+ Cabela's Big Game Hunter PS2 T Call of Duty 3 Wii T Captain America, Super Soldier PS3 T Crash Bandicoot N Sane Trilogy PS4 E10+ Crew 2 PS4, XboxOne T Dance Central 3 Xbox 360 T De Blob 2 Xbox 360 E Dead Cells PS4 T Deadly Creatures Wii T Deca Sports 3 Wii E Deformers: Ready at Dawn PS4, XboxOne E10+ Destiny PS3, Xbox 360 T Destiny 2 PS4, XboxOne T Dirt 4 PS4, XboxOne T Dirt Rally 2.0 PS4, XboxOne E Donkey Kong Country Returns Wii E Don't Starve Mega Pack PS4, XboxOne T Dragon Quest 11 PS4 T Highland Township Public Library - Video Game Collection Updated January 2020 Game Console Rating Dragon Quest Builders PS4 E10+ Dragon -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor.