Glycemic Index, Glycemic Load and Their Association with Glycemic Control Among Patients with Type 2 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations
nutrients Review Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations Geoffrey Livesey 1,* , Richard Taylor 1, Helen F. Livesey 1, Anette E. Buyken 2, David J. A. Jenkins 3,4,5,6, Livia S. A. Augustin 4,7, John L. Sievenpiper 3,4,5,6, Alan W. Barclay 8, Simin Liu 9, Thomas M. S. Wolever 3,4, Walter C. Willett 10, Furio Brighenti 11 , Jordi Salas-Salvadó 12,13 , Inger Björck 14, Salwa W. Rizkalla 15, Gabriele Riccardi 16, Carlo La Vecchia 17 , Antonio Ceriello 18, Antonia Trichopoulou 19, Andrea Poli 20, Arne Astrup 21 , Cyril W. C. Kendall 3,4,22, Marie-Ann Ha 23 , Sara Baer-Sinnott 24 and Jennie C. Brand-Miller 25 1 Independent Nutrition Logic Ltd, 21 Bellrope Lane, Wymondham NR180QX, UK; [email protected] (R.T.); [email protected] (H.F.L.) 2 Institute of Nutrition, Consumption and Health, Faculty of Natural Sciences, Paderborn University, 33098 Paderborn, Germany; [email protected] 3 Departments of Nutritional Science and Medicine, Faculty of Medicine, University of Toronto, Toronto, ON M5S 1A8, Canada; [email protected] (D.J.A.J.); [email protected] (J.L.S.); [email protected] (T.M.S.W.); [email protected] (C.W.C.K.) 4 Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada; [email protected] 5 Division of Endocrinology and Metabolism, Department of Medicine, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada 6 Li Ka Shing Knowledge Institute, St. -

A Dozen Tips for Better Blood Sugar Control: Glycemic Index And
A Dozen Tips for Better Blood Sugar Control: Glycemic Index and Glycemic Load Human bodies depend on a steady supply of glucose (blood sugar) as their principal fuel, in order for muscles to stretch and contract, nerves to fire, brains to function—and so much more. Glucose comes from carbohydrates, so the quality and quantity of carbohydrates we eat hugely impacts our energy levels and overall health. Too little glucose, and we starve many bodily functions (especially the brain, which uses 11–20% of the glucose we produce). Too much, and our body scrambles to produce enough insulin to process all that blood sugar—and we may develop heart disease, eye, kidney, and nerve damage. Ideally, our food delivers a steady stream of just the right amount of glucose. But how do we distinguish foods, meals, and diets that raise our blood sugar too high and too fast from those that dole out their fuel slowly and steadily to support good health? Understanding glycemic index, glycemic load and glycemic response can help. Glycemic Index (GI) ranks the quality of individual carbohydrate-rich foods on a scale of 1–100 by measuring how glucose levels rise after someone eats an amount of that food containing 50 grams of carbohydrate. Foods with a low GI score (under 55) provide steady fuel to support energy levels and overall health, while those with a high GI score (70 and up) are likely to provide an unhealthy quick rush of blood sugar followed by a sharp crash. Glycemic Load (GL) combines quality and quantity, allowing us to rank how the typical serving size of a food affects blood sugar. -

POTATOES and the GLYCEMIC INDEX How to Reduce the GI of Potatoes and Gain the Benefits of Resistant Starch
POTATOES AND THE GLYCEMIC INDEX How to reduce the GI of potatoes and gain the benefits of resistant starch Potatoes are a highly nutritious wholefood. They contribute a range of nutrients associated with good health, including fibre, vitamin B6, vitamin C, potassium and phytonutrients. You might also be surprised to know that potatoes can have a lower glycemic index (GI) than you might think. What is the Glycemic Index? The GI is a measure of the speed at which a food makes blood sugar rise. AT A GLANCE GI is calculated by measuring the change in blood glucose (BGL) over a The GI of potatoes is two-hour period after eating a 50 gram carbohydrate portion of a single food relative to the change in blood glucose after eating a 50g carbohydrate influenced by: portion of a reference food (glucose or white bread). • Variety of potato (and its carbohydrate, fibre and When it is not possible to measure this directly, GI tables are used to provide polyphenol content) an estimate of BGL response, however, there are large fluctuations in BGL response to foods both within and between individuals. • Serving size The lower a food’s GI, the slower BGLs rise after eating that food. There are • Cooking method/preparation many factors affecting GI in nuts and potatoes. GI less than 55 is considered and storage low, 56-69 moderate and 70+ high. • Fibre and resistant starch content (higher amounts = To better understand a food’s effect on BGLs, you need to know how quickly lower GI, leave the skin on) its glucose enters the bloodstream and how much glucose per serving it can deliver. -

Low–Glycemic Index Diets in the Management of Diabetes a Meta-Analysis of Randomized Controlled Trials
Clinical Care/Education/Nutrition ORIGINAL ARTICLE Low–Glycemic Index Diets in the Management of Diabetes A meta-analysis of randomized controlled trials 1 2 JENNIE BRAND-MILLER, PHD PETER PETOCZ, PHD ticularly with regard to the glycemic in- 2 3 SUSAN HAYNE, BSC STEPHEN COLAGIURI, MD dex (GI) of foods (5). Current dietary recommendations emphasize the quantity rather than the quality of carbohydrate, despite the fact that carbohydrate source and nature pro- OBJECTIVE — The use of diets with low glycemic index (GI) in the management of diabetes foundly influence postprandial glycemia is controversial, with contrasting recommendations around the world. We performed a meta- (6,7). Research on GI indicates that even analysis of randomized controlled trials to determine whether low-GI diets, compared with conventional or high-GI diets, improved overall glycemic control in individuals with diabetes, as when foods contain the same amount of assessed by reduced HbA or fructosamine levels. carbohydrate (i.e., carbohydrate ex- 1c changes), there are up to fivefold differ- RESEARCH DESIGN AND METHODS — Literature searches identified 14 studies, ences in glycemic impact (8). In addition, comprising 356 subjects, that met strict inclusion criteria. All were randomized crossover or several prospective observational studies parallel experimental design of 12 days’ to 12 months’ duration (mean 10 weeks) with modifi- have found that the overall GI and glyce- cation of at least two meals per day. Only 10 studies documented differences in postprandial mic load (GI ϫ g carbohydrate) of the glycemia on the two types of diet. diet, but not total carbohydrate content, are independently related to the risk of RESULTS — Low-GI diets reduced HbA by 0.43% points (CI 0.72–0.13) over and above 1c developing type 2 diabetes (9,10), cardio- that produced by high-GI diets. -

Dietary Patterns, Foods and Nutrients, and Health Outcomes What Is The
Appendix E-2.27: Evidence Portfolio Appendix E2.27: Evidence Portfolio Part D. Chapter 2: Dietary Patterns, Foods and Nutrients, and Health Outcomes What is the relationship between dietary patterns and measures of body weight or obesity? Conclusion Statement: The DGAC concurs with the 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity1 that strong evidence demonstrates that, preferably as part of a comprehensive lifestyle intervention carried out by multidisciplinary teams of professionals or nutrition professionals, overweight and obese adults can achieve weight loss through a variety of dietary patterns that achieve an energy deficit. Clinically meaningful weight losses that were achieved ranged from 4 to 12 kg at 6-month follow-up. Thereafter, slow weight regain is observed, with total weight loss at 1 year of 4 to 10 kg and at 2 years of 3 to 4 kg. However, some dietary patterns may be more beneficial in the long-term for cardiometabolic health. DGAC Grade: Strong The DGAC concurs with the NEL Dietary Patterns Systematic Review Project2 that moderate evidence indicates dietary patterns that are higher in vegetables, fruits, and whole grains; include seafood and legumes; are moderate in dairy products (particularly low and non-fat dairy) and alcohol; lower in meats (including red and processed meats), and low in sugar-sweetened foods and beverages, and refined grains are associated with favorable outcomes related to healthy body weight (including lower BMI, waist circumference, or percent body fat) or risk of obesity. Components of the dietary patterns associated with these favorable outcomes include higher intakes of unsaturated fats and lower intakes of saturated fats, cholesterol, and sodium. -

A Review of the Relationship Between Dietary Glycemic Index and Glycemic Load and Type 2 Diabetes
eISSN: 2476-7425 pISSN: 2476-7417 JNFS 2016; 1 (1): 73-79 Website: jnfs.ssu.ac.ir A Review of the Relationship between Dietary Glycemic Index and Glycemic Load and Type 2 Diabetes 1,2 3 4 Fahime Zeinali; MSc , Hadis Asadi Samani; BSc , Omid Toupchian; PhD , Shima Abdollahi; MSc1,2 & Mehnoosh Samadi; MSc*3 1 Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 2 Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 3 Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 4 Department of Cellular and Molecular Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran. ARTICLE INFO ABSTRACT REVIEW ARTICLE Background: In recent decades, studies on type 2 diabetes (T2D), have adopted a new approach to the field of a more complete collection of variables Article history: related to the lifestyles and diet of people. Diet is an important factor in Received: 9 Apr 2016 increasing the rate of T2D among individuals. Considering the consumption of Revised: 5 Jun 2016 a high-carbohydrate diet, little attention has been paid to the type of Accepted: 24 Jul 2016 carbohydrates consumed in the incidence of T2D. The present study aimed to review the literature on the relationship between the glycemic index (GI), the glycemic load, (GL) and T2D, it also targets at evaluating and comparing the *Corresponding author: results of similar studies in other countries Methods: Using search engines, Downloaded from jnfs.ssu.ac.ir at 11:48 IRST on Saturday October 2nd 2021 Student Research Committee, including PubMed, Science Direct, Embase and Scopus, and key words such Ahvaz Jundishapur University as GI, GL, diabetes; articles with cross-sectional, clinical trial, Prospective and of Medical Sciences, Ahvaz, retrospective cohort designs between 2000 to 2016 were selected. -
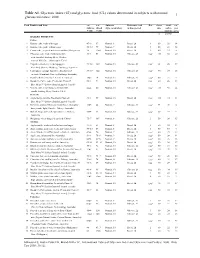
And Glycemic Load (GL) Values Determined in Subjects with Normal 1 Glucose Tolerance: 2008
Table A1. Glycemic index (GI) and glycemic load (GL) values determined in subjects with normal 1 glucose tolerance: 2008 Food Number and Item GI2 GI2 Subjects Reference food Ref. Serve Avail. GL3 (Glucose (Bread (type & number) & time period Size carbo- per = 100) = 100) hydrate serve g g/serve BAKERY PRODUCTS Cakes 1 Banana cake, made with sugar 47±8 67 Normal, 8 Bread, 2h 1 60 29 14 2 Banana cake, made without sugar 55±10 79 Normal, 7 Bread, 2h 1 60 22 12 3 Carrot cake, prepared with coconut flour (Philippines) 36 52±3 Normal, 10 Bread, 2h 2 60 23 8 4 Chocolate cake made from packet mix 38±3 54 Normal, 10 Glucose, 2h UO4 111 52 20 with chocolate frosting (Betty Crocker, General Mills Inc., Minneapolis, USA) 5 Cupcake, strawberry-iced (Squiggles, 73±12 104 Normal, 10 Glucose, 2h UO4 38 26 19 Farmland, Grocery Holdings, Tooronga, Australia) 6 Lamingtons (sponge dipped in chocolate and 87±17 124 Normal, 10 Glucose, 2h UO4 50 29 25 coconut) (Farmland, Grocery Holdings, Australia) 7 Pound cake 0% (Bimbo S.A de C.V, Mexico) 38±5 54 Normal, 12 Glucose, 2h UO4 60 25 9 8 Raspberry Coffee cake, President's Choice® 50±4 71 Normal, 10 Bread, 2h UO5 60 26 13 Blue Menu™ (Loblaw Brands Limited, Canada) 9 Vanilla cake, made from packet mix with 42±4 60 Normal, 10 Glucose, 2h UO4 111 58 24 vanilla frosting (Betty Crocker, USA) Desserts 10 Apple Berry crumble, President's Choice® 41±3 59 Normal, 10 Bread, 2h UO5 165 34 14 Blue Menu™ (Loblaw Brands Limited, Canada) 11 Bavarian (mousse filling on biscuit base), Chocolate 31±5 44 Normal, 9 Glucose, 2h UO6 -

GLYCEMIC LOAD the Glycemic Load (GL) Is a Ranking System for Carbohydrate Content in Food Portions Based on Their Glycemic Index
GLYCEMIC LOAD From Wikipedia, the free encyclopedia The glycemic load (GL) is a ranking system for carbohydrate content in food portions based on their glycemic index (GI) and a standardized portion size of 100g. Glycemic load or GL combines both the quality and quantity of carbohydrate in one number. It is the best way to predict blood glucose values of different types and amounts of food. The formula is: GL = GI x the amount of available carbohydrate in a 100g serving / 100. The glycemic load assesses the impact of carbohydrate consumption using the glycemic index but takes into account the amount of carbohydrate that is consumed. GL is a weighted GI value. For instance, watermelon has a high GI – but watermelon does not actually contain much carbohydrate, so the glycemic effect of eating it (and therefore its GL) is actually relatively low. A GL greater than 20 is considered high, a GL of 11-19 is considered medium, and a GL of 10 or less is considered low. Foods that have a low GL almost always have a low GI. Foods with an intermediate or high range GL range from a very low to very high GI. The rationale for establishing glycemic load as an important characteristic of a food is based on the fact that a larger amount of a food with certain glycemic index that contains few available carbohydrates would have the same effect on blood sugar as a smaller amount of a food that has the same glycemic index but a higher carbohydrate content. Glycemic Load is the product of the Glycemic Index and the grams of available carbohydrate in a 100g serving (GL = GI × available Carb grams). -

Potatoes and the Glycemic Index
NUTRITION LIBRARY GLYCEMIC INDEX POTATOES AND THE GLYCEMIC INDEX The health-promoting benefits of vegetables are well-documented in scientific literature and potatoes have long held the prominent position of America’s favorite vegetable for their versatility, taste and nutritional value. So why have so many diet books, popular magazines and self-proclaimed “nutritionists” told the American public to stop eating potatoes? They have been lured into believing that a controversial physiological measure known as the glycemic index (GI) is the silver bullet to managing weight and preventing disease. Originally developed as a tool for the dietary management of diabetes,1 the GI has been promoted to the general public as a dietary tool for weight loss and disease prevention, despite a lack of scientific support for such uses. GLYCEMIC INDEX DEFINED chronic diseases including diabetes, heart disease, 4 Almost all carbohydrates, regardless of the form and even colon cancer. In fact, despite the media in which they are consumed (e.g., starch, lactose, hype, scientific evidence largely supports consuming a sucrose) are digested to glucose which then enters the moderate-to-high carbohydrate diet to prevent obesity 5 bloodstream, causing a temporary rise in blood glucose and decrease the risk of chronic disease. levels. This rise in blood glucose levels is referred to as the “glycemic response.” The glycemic index (GI) A GLYCEMIC GRAY AREA is a mathematical calculation that takes into account A number of factors have been shown to affect the GI an individual’s glycemic response to a test food of a food, rendering the usefulness and practicality of (e.g., potatoes) relative to the glycemic response to the GI in diet planning questionable at best.5 reference food (typically either white bread or glucose). -

Low-Glycemic Index Diets As an Intervention for Diabetes: a Systematic Review and Meta-Analysis
Downloaded from https://academic.oup.com/ajcn/article-abstract/110/4/891/5543221 by ASN Member Access user on 02 January 2020 See corresponding editorial on page 921. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis Mohammad Ishraq Zafar,1 Kerry E Mills,2 Juan Zheng,1 Anita Regmi,1 Sheng Qing Hu,1 Luoning Gou,1 and Lu-Lu Chen1 1Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P.R. China and 2Faculty of Science and Technology, University of Canberra, Canberra, Australia ABSTRACT compared with glucose or white bread. The glycemic load Background: Low-glycemic index (GI) diets are thought to reduce calculates the amount that the whole food raises blood glucose postprandial glycemia, resulting in more stable blood glucose postprandially (2). Low-GI foods, such as fructose, lower peak concentrations. postprandial blood glucose excursions (3) and have been shown Objective: We hypothesized that low-GI diets would be superior to to have a positive effect on glucose control (4). A recent network other diet types in lowering measures of blood glucose control in meta-analysis including 56 trials showed that various dietary people with type 1 or type 2 diabetes, or impaired glucose tolerance. types lowered both glycated hemoglobin (HbA1c) and fasting Methods: We searched PubMed, the Cochrane Library, EMBASE, blood glucose (FBG) compared with a control diet (5), and and clinical trials registries for published and unpublished studies up another meta-analysis of 18 trials showed similar results when until 1 March, 2019. -

Glycemic Index Trends and Clinical Implications: Where Are We Going?
Revista Latinoamericana de Hipertensión ISSN: 1856-4550 [email protected] Sociedad Latinoamericana de Hipertensión Venezuela Glycemic Index Trends and Clinical Implications: Where Are We Going? Angarita Dávila, Lisse; Escobar Contreras, Ma. Cristina; Durán Agüero, Samuel; Céspedes Nava, Virginia; Guerrero-Wyss, Maryon; de Assis Costa, Jorge; Bermúdez Pirela, Valmore Glycemic Index Trends and Clinical Implications: Where Are We Going? Revista Latinoamericana de Hipertensión, vol. 13, no. 6, 2018 Sociedad Latinoamericana de Hipertensión, Venezuela Available in: https://www.redalyc.org/articulo.oa?id=170263777020 Derechos reservados. Queda prohibida la reproducción total o parcial de todo el material contenido en la revista sin el consentimiento por escrito del editor en jefe. Copias de los artículos: Todo pedido de separatas deberá ser gestionado directamente con el editor en jefe, quien gestionará dicha solicitud ante la editorial encargada de la publicación. This work is licensed under Creative Commons Attribution-NoDerivs 4.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Lisse Angarita Dávila, et al. Glycemic Index Trends and Clinical Implications: Where Are We Going? Artículos Glycemic Index Trends and Clinical Implications: Where Are We Going? Índice Glicémico: Tendencias e implicaciones clínicas ¿Hacia dónde vamos? Lisse Angarita Dávila Redalyc: https://www.redalyc.org/articulo.oa? Escuela de Nutrición y Dietética, Facultad de Medicina, id=170263777020 Universidad Andres Bello, Sede Concepción, Talcahuano, Chile., Chile [email protected] http://orcid.org/0000-0001-7860-5112 Ma. Cristina Escobar Contreras Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Sede Concepción, Talcahuano, Chile., Chile http://orcid.org/0000-0002-8474-5573 Samuel Durán Agüero Escuela de Nutrición y Dietética, Facultad de Ciencias para el Cuidado de la Salud, Universidad San Sebastián, Santiago, Lota 2465, Santiago 8320000. -
Glycemic Index and Glycemic Load for 100+ Foods Measuring Carbohydrate E!Ects Can Help Glucose Management
CART FREE HEALTHBEAT SIGNUP SHOP ▼ SIGN IN What can we help you find? HEART HEALTH MIND & MOOD PAIN STAYING CANCER DISEASES & MEN'S HEALTH WOMEN'S HEALTHY CONDITIONS HEALTH Glycemic index and glycemic load for 100+ foods Measuring carbohydrate e!ects can help glucose management Updated: August 27, 2015 Published: February, 2015 The glycemic index is a value assigned to foods based on how slowly or how quickly those foods cause increases in blood glucose levels. Also known as "blood sugar," blood glucose levels above normal are toxic and can cause blindness, kidney failure, or increase cardiovascular risk. Foods low on the glycemic index (GI) scale tend to release glucose slowly and steadily. Foods high on the glycemic index release glucose rapidly. Low GI foods tend to foster weight loss, while foods high on the GI scale help with energy recovery after exercise, or to offset hypo- (or insufficient) glycemia. Long-distance runners would tend to favor foods high on the glycemic index, while people with pre- or full-blown diabetes would need to concentrate on low GI foods. Why? People with diabetes can't produce sufficient quantities of insulin—which helps process blood sugar—which means they are likely to have an excess of blood glucose. The slow and steady release of glucose in low-glycemic foods is helpful in keeping blood glucose under control. But the glycemic index of foods tells only part of the story. What it doesn't tell you is how high your blood sugar could go when you actually eat the food, which is partly determined by how much carbohydrate is in an individual serving.