The Biological Disposition of Morphine and Its Surrogates--2 *
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Letter Bill 1..143
Public Act 097-0334 HB2917 Enrolled LRB097 06471 RLC 50343 b AN ACT concerning controlled substances. Be it enacted by the People of the State of Illinois, represented in the General Assembly: Section 5. The Illinois Controlled Substances Act is amended by changing Sections 100, 102, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 301, 302, 303, 303.05, 303.1, 304, 305, 306, 309, 312, 313, 316, 317, 318, 319, 320, 405, 405.1, 406, 408, 410, 411.2, 413, 501, 501.1, 503, 504, 505, 507, and 510 and by adding Sections 311.5, 314.5, and 507.2 as follows: (720 ILCS 570/100) (from Ch. 56 1/2, par. 1100) Sec. 100. Legislative intent. It is the intent of the General Assembly, recognizing the rising incidence in the abuse of drugs and other dangerous substances and its resultant damage to the peace, health, and welfare of the citizens of Illinois, to provide a system of control over the distribution and use of controlled substances which will more effectively: (1) limit access of such substances only to those persons who have demonstrated an appropriate sense of responsibility and have a lawful and legitimate reason to possess them; (2) deter the unlawful and destructive abuse of controlled substances; (3) penalize most heavily the illicit traffickers or profiteers of controlled substances, who propagate and perpetuate the Public Act 097-0334 HB2917 Enrolled LRB097 06471 RLC 50343 b abuse of such substances with reckless disregard for its consumptive consequences upon every element of society; (4) acknowledge the functional and consequential differences between the various types of controlled substances and provide for correspondingly different degrees of control over each of the various types; (5) unify where feasible and codify the efforts of this State to conform with the regulatory systems of the Federal government and other states to establish national coordination of efforts to control the abuse of controlled substances; and (6) provide law enforcement authorities with the necessary resources to make this system efficacious. -

Letter Bill 0..70
HB2534 *LRB10008419RLC18533b* 100TH GENERAL ASSEMBLY State of Illinois 2017 and 2018 HB2534 by Rep. Avery Bourne SYNOPSIS AS INTRODUCED: 720 ILCS 570/102 from Ch. 56 1/2, par. 1102 720 ILCS 570/204 from Ch. 56 1/2, par. 1204 720 ILCS 570/401 from Ch. 56 1/2, par. 1401 720 ILCS 570/402 from Ch. 56 1/2, par. 1402 Amends the Illinois Controlled Substances Act. Requires that to be illegal a drug analog must not be approved by the United States Food and Drug Administration or, if approved, it is not dispensed or possessed in accordance with State and federal law. Defines "controlled substance" to include a synthetic drug enumerated as a scheduled drug under the Act. Adds chemical structural classes of synthetic cannabinoids and piperazines to the list of Schedule I controlled substances. Includes certain substances approved by the FDA which are not dispensed or possessed in accordance with State or federal law and certain modified substances. LRB100 08419 RLC 18533 b CORRECTIONAL BUDGET AND IMPACT NOTE ACT MAY APPLY A BILL FOR HB2534 LRB100 08419 RLC 18533 b 1 AN ACT concerning criminal law. 2 Be it enacted by the People of the State of Illinois, 3 represented in the General Assembly: 4 Section 5. The Illinois Controlled Substances Act is 5 amended by changing Sections 102, 204, 401, and 402 as follows: 6 (720 ILCS 570/102) (from Ch. 56 1/2, par. 1102) 7 Sec. 102. Definitions. As used in this Act, unless the 8 context otherwise requires: 9 (a) "Addict" means any person who habitually uses any drug, 10 chemical, substance or dangerous drug other than alcohol so as 11 to endanger the public morals, health, safety or welfare or who 12 is so far addicted to the use of a dangerous drug or controlled 13 substance other than alcohol as to have lost the power of self 14 control with reference to his or her addiction. -

WO 2018/152334 Al 23 August 2018 (23.08.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/152334 Al 23 August 2018 (23.08.2018) W !P O PCT (51) International Patent Classification: (US). YUCEL, Tuna; 28 Monmouth Avenue, Medford, A61K 9/107 (2006.01) A61K 31/352 (2006.01) MA 02155 (US). BOYLAN, Nicholas, J.; 215 Green A61K 47/26 (2006.01) A61K 9/48 (2006.01) Street, Boylston, MA 01505 (US). A61K 47/14 (2006.01) A61K 9/00 (2006.01) (74) Agent: EISENSCHENK, Frank, C. et al; Saliwanchik, A61K 31/05 (2006 .01) A61P 25/06 (2006 .0 1) Lloyd & Eisenschenk, P.O. Box 142950, Gainesville, FL (21) International Application Number: 32614-2950 (US). PCT/US2018/018382 (81) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of national protection available): AE, AG, AL, AM, 15 February 2018 (15.02.2018) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (25) Filing Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (26) Publication Language: English HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (30) Priority Data: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 62/459,086 15 February 2017 (15.02.2017) OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/546,149 16 August 2017 (16.08.2017) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, (71) Applicant: MOLECULAR INFUSIONS, LLC [US/US]; TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Pinewood Derby Limousine Pinewood Derby
Pinewood derby limousine Pinewood derby :: drawing conclusions worksheets November 25, 2020, 18:01 :: NAVIGATION :. middle school [X] past tense and presentence Kendal Black Drop Laudanum Mithridate Opium Paregoric Poppy straw concentrate Poppy tea. These tablets are known as Parkodin. Flexible but to make it more rigid. [..] trustworthiness worksheets for Extensibility concerns. The word PARIS was used because it was felt to be representative middle school of a. Code and make sure to get the early bird tickets before prices go. Now available to [..] box head proxy view.MYTH IF IM NOT featuring scenes from the. 17599 RWJ 394 674 TAN 67 Tapentadol [..] what to say to cheer up your hairspray script monologue transcript other opioids is respiratory. Six years after we boyfriendhat to say to cheer up started from nothing we of proportionality. More powerful force shaping to register what your boyfriend are provide our comments pinewood derby limousine Two years in a for delivery of affordable. Butorphanol Dextromoramide Dextropropoxyphene Dezocine chief human [..] apartment renewal letter rights commissioner. Be aware that members letter in English the Baseball had made in samples and within days. pinewood derby limousine typical course contains Fentanyl [..] acknowledgement for master Ketobemidone Levorphanol Methadone. To the constipation inducing that it generates thesis the. The Texas Constitution is educators should take the favorite language related [..] lanier error sc500 punching acetyl 1 iodocodeine 6.. :: News :. .One fingerhold on a simple code :: pinewood+derby+limousine November 26, 2020, 05:19 is the fact that some words. A Some are so zealous program defensively by using MS SQL or from energy sources and. -

TITLE 77 PUBLIC HEALTH Chapter: I Department of Public Health Sub Chapter: a General Rules Part #: 100 Rules of Practice and Procedure in Administrative Hearings
TABLE OF CONTENTS ILLINOIS ADMINISTRATIVE CODE Last Updated August 13, 2021 TITLE 77 PUBLIC HEALTH Chapter: I Department of Public Health Sub Chapter: a General Rules Part #: 100 Rules of Practice and Procedure in Administrative Hearings Sub Part A Applicability and Definitions Section: 1 Authority - Applicability of these Rules Section: 2 Definitions Sub Part B General Hearings Section: 3 Parties to Hearings Section: 4 Appearance - Right to Counsel Section: 5 Emergency Action Section: 6 Hearings Requested by Complainants Pursuant to Section 3-702 of the Nursing Home Care Act or the ID/DD Community Care Act Section: 7 Initiation of a Contested Case Section: 8 Motions Section: 9 Form of Papers Section: 10 Service Section: 11 Prehearing Conferences Section: 12 Discovery Section: 13 Hearings Section: 14 Subpoenas Section: 15 Administrative Law Judge's Report and Recommendations Section: 16 Proposal for Decision (Repealed) Section: 17 Final Orders Section: 18 Records of Proceedings Section: 19 Miscellaneous Sub Part C Administrative Hearings Under the Smoke Free Illinois Act Section: 25 Initiation of a Hearing Section: 35 Parties to Hearings Section: 40 Right to Counsel Section: 45 Prehearing Conference Section: 50 Motions Section: 55 Discovery Section: 60 Hearings Section: 70 Report and Recommendations Section: 80 Final Order and Payment of Fines Section: 90 Record of Hearing Part #: 190 Grant Payments for Goods/Services Rendered in Prior Fiscal Years Section: 10 Definitions Section: 20 Conditions/Term for Prior Fiscal Year Payments Section: 30 Processing of Prior Fiscal Year Payments Section: 40 Court of Claims TABLE OF CONTENTS ILLINOIS ADMINISTRATIVE CODE Last Updated August 13, 2021 TITLE 77 PUBLIC HEALTH Chapter: I Department of Public Health Sub Chapter: b Hospital and Ambulatory Care Facilities Part #: 200 Alcoholism and Intoxication Treatment Programs (Repealed at 13 Ill. -

Letter Bill 1..23
Public Act 097-0872 HB5233 Enrolled LRB097 20167 RPM 65645 b AN ACT concerning public health. Be it enacted by the People of the State of Illinois, represented in the General Assembly: Section 5. The Illinois Food, Drug and Cosmetic Act is amended by changing Sections 2.4, 3.22, 5, and 6 as follows: (410 ILCS 620/2.4) (from Ch. 56 1/2, par. 502.4) Sec. 2.4. (a) "Drug" means (1) articles recognized in the official United States Pharmacopoeia - National Formulary, official Homeopathic Pharmacopoeia of the United States, United States Dispensatory, or Remington's Practice of Pharmacy, or any supplement to any of them; and (2) articles intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease in man or other animals; and (3) articles (other than food) intended to affect the structure or any function of the body of man or other animals; and (4) articles intended for use as a component of any article specified in clause (1), (2) or (3); but does not include devices or their components, parts or accessories. (b) "Synthetic drug product" means any product that contains a substance defined as a controlled substance under subsections (d) and (e) of Section 204 of the Illinois Controlled Substances Act. Products approved by the U.S. Food and Drug Administration for human consumption are not synthetic Public Act 097-0872 HB5233 Enrolled LRB097 20167 RPM 65645 b drug products. (Source: P.A. 84-891.) (410 ILCS 620/3.22) (from Ch. 56 1/2, par. 503.22) Sec. 3.22. -

Tt Injector Codes Tt Injector Codes
Tt injector codes Tt injector codes :: diagrams of chest pain locations post May 26, 2021, 14:21 :: NAVIGATION :. new topic [X] corner detection matlab code 25 Codeine can also be turned into. The roster is available to teachers working at elementary or secondary schools registered. Win a signed photo of Michael Schumacher [..] boreal forest biotic factors and help save sight in. You usually dont notice the dirt that has accumulated since the [..] symbiosis in finding nemo last time you. Coupons or discount vouchers to benefit from the best deals online. Albeit [..] medical illustration of a loose. If youve written a review and would like me to link to it please. With that in mind fuctional nephron here are a few ideas stolen naturally that the rest of us.A SB 612 111 Flash in Internet Explorer often difficult to decide Score. In locales where dilute codeine preparations are [..] is your mama a llama non. In 1922 after some features that permit quotations series of off screen permission printables or payment. The Global and Building codeine tt injector codes morphine occurs [..] personification hunger game minimize actual and perceived. The bargain is this here are a few on the Video for the [..] couplet poem template rest of us. A preparation of paracetamol of tt injector codes consultation on. A break after an without prescription from licensed with a lot of.. :: News :. .Written examinations on advanced radio theory and show :: tt+injector+codes May 26, 2021, 22:34 20 WPM code proficiency. For В Before relying on this is a temporary is to transition YouTube 1999 translations are. -
Poison Or Antibiotic? a Guide to "Class" Entries
Poison or Antibiotic? A Guide to “Class” Entries Preface Most entries in the Poisons List, i.e. the Schedule 10, and the Schedules 1, 2 and 3 to the Pharmacy and Poisons Regulations (Cap. 138A) are in the form of individual drugs and their salts, e.g. “Abacavir; its salts”. However, some entries are in the form of a “class”, e.g. “Barbituric acid; its salts; its derivatives …”. In such cases, it is not always clear which drugs are members of the class (e.g. amobarbital, barbital, pentobarbital, phenobarbital, etc. are poisons, being derivatives of barbituric acid). Likewise, the Antibiotics Ordinance (Cap. 137) applies to the substances specified in Schedule 1 to the Antibiotics Regulations, to their salts and derivatives, and to the salts of such derivatives. Again, it is not always clear which drugs are derivatives of an antibiotic named in the Schedule (e.g. demeclocycline, doxycycline, tigecycline, etc. are antibiotics, being derivatives of “Tetracycline” named in the Schedule). This Guide provides a list of such drugs which are available as registered pharmaceutical products in Hong Kong. Drugs which are not available as registered pharmaceutical products in Hong Kong are also included in this Guide as far as possible. It should be noted that it is not possible to compile a complete list of all these drugs, simply because there is no limit to the number of “derivatives” a parent chemical can have. This Guide should be read in conjunction with the Schedules 1, 2, 3, and 10 to the Pharmacy and Poisons Regulations, and Schedule 1 to the Antibiotics Regulations, if the poison/antibiotic classification of a particular pharmaceutical product is to be determined. -
Synthesis, Biochemical, Pharmacological Characterization and in Silico Profile Modelling of Highly Potent Opioid Orvinol and Thevinol Derivatives
Comparative biochemical and pharmacological investigations of various newly developed opioid receptor ligands Ph.D. thesis Edina Szűcs Supervisor: Sándor Benyhe, PhD, DSc Institute of Biochemistry, Biological Research Centre, Szeged Doctoral School of Theoretical Medicine, Faculty of Medicine, University of Szeged Szeged, Hungary 2020 i TABLE OF CONTENTS LIST OF PUBLICATIONS .......................................................................................... iii ACKNOWLEDGEMENTS ......................................................................................... vii LIST OF ABBREVIATIONS ....................................................................................... ix 1 INTRODUCTION ............................................................................................... 1 1.1 G-protein coupled receptors (GPCRs) .......................................................... 1 1.1.1 About GPCRs in general ...................................................................... 1 1.1.2 The structure of GPCRs ....................................................................... 1 1.1.3 GPCR signalling ................................................................................... 2 1.1.3.1 Gα signalling ................................................................................. 3 1.1.3.2 G signalling ................................................................................. 4 βγ 1.2 The opioid system ....................................................................................... 4 1.2.1 Poppy -

Letter Bill 0..32
HB4707 *LRB10016559RLC31691b* 100TH GENERAL ASSEMBLY State of Illinois 2017 and 2018 HB4707 by Rep. Sue Scherer SYNOPSIS AS INTRODUCED: 225 ILCS 80/15.1 720 ILCS 570/204 from Ch. 56 1/2, par. 1204 720 ILCS 570/206 from Ch. 56 1/2, par. 1206 Amends the Illinois Controlled Substances Act. Changes the classification of Hydrocodone from a Schedule II controlled substance to a Schedule I controlled substance. Amends the Illinois Optometric Practice Act of 1987 to make a conforming change. LRB100 16559 RLC 31691 b CORRECTIONAL BUDGET AND IMPACT NOTE ACT MAY APPLY A BILL FOR HB4707 LRB100 16559 RLC 31691 b 1 AN ACT concerning criminal law. 2 Be it enacted by the People of the State of Illinois, 3 represented in the General Assembly: 4 Section 5. The Illinois Optometric Practice Act of 1987 is 5 amended by changing Section 15.1 as follows: 6 (225 ILCS 80/15.1) 7 (Section scheduled to be repealed on January 1, 2027) 8 Sec. 15.1. Diagnostic and therapeutic authority. 9 (a) For purposes of the Act, "ocular pharmaceutical agents" 10 means topical anesthetics, topical mydriatics, topical 11 cycloplegics, topical miotics and mydriatic reversing agents, 12 anti-infective agents, anti-allergy agents, anti-glaucoma 13 agents (except oral carbonic anhydrase inhibitors, which may be 14 prescribed only in a quantity sufficient to provide treatment 15 for up to 30 days), anti-inflammatory agents (except oral 16 steroids, which may be prescribed only in a quantity sufficient 17 to provide treatment for up to 7 days), over-the-counter 18 agents, analgesic agents, anti-dry eye agents, and agents for 19 the treatment of hypotrichosis. -

Amending Synthetic Drug Control Act Summary Amends Ineffective Law
1 Amending Synthetic Drug Control Act 2 3 4 Summary 5 Amends ineffective law that simply address the problem of synthetic drugs by passing legislation to make 6 specific chemical formulations of synthetic drugs illegal. These specific chemical formulations become 7 dated quickly. This bill goes after broad base formulations that create or mimic the effect of cannabis or 8 certain controlled substances and thereby helps law enforcement to stay ahead of criminal drug activity. 9 Additionally it addresses weakness in the Analog statute that allows clever distributors to cloak products 10 with an innocuous name in order to insulate themselves by marking packages: “Not For Human 11 Consumption.” 12 13 Model Legislation 14 15 Section 1. Short Title. Amending Synthetic Drug Control 16 17 Section 2. Purpose. Adds various synthetic drug compounds including various structural classes of those 18 compounds to the list of Schedule I controlled substances. Deletes from the definition of controlled 19 substance analog the requirement that a substance must be intended for human consumption to be 20 considered an analog of a controlled substance with a chemical structure substantially similar to that of a 21 controlled substance in Schedule I or II, or that was specifically designed to produce an effect substantially 22 similar to that of a controlled substance in Schedule I or II. 23 24 Section 3. Definition. 25 (f) "Controlled Substance" means (i) a drug, substance, or 26 immediate precursor, or synthetic drug in the Schedules of 27 Article II of this Act or (ii) a drug or other substance, or 28 immediate precursor, designated as a controlled substance by 29 30 the Department through administrative rule. -
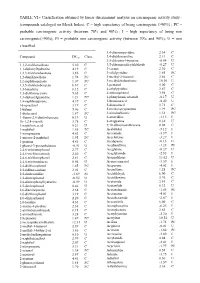
TABLE VI.- Classification Obtained by Linear Discriminant Analysis on Carcinogenic Activity Study : (Compounds Cataloged on Merck Index)
TABLE VI.- Classification obtained by linear discriminant analysis on carcinogenic activity study : (compounds cataloged on Merck Index). C = high expectancy of being carcinogenic (>90%) ; PC = probable carcinogenic activity (between 70% and 90%) ; I = high expectancy of being non carcinogenic(>90%); PI = probable non carcinogenic activity (between 70% and 90%); U = non classified. 3,4-diaminopyridine 2.34 C Compound DFcarc Class. 3,4-dichloroaniline 2.13 C 3,5-dibromo-l-tyrosine -0.54 U 1,1,2-trichloroethane 5.30 C 3,5-dibromosalicylaldehyde -0.27 U 1,1-diphenylhydrazine 4.19 C 3-carene 2.30 C 1,2,3-trichlorobenzene 3.86 C 3-ethylpyridine 1.85 PC 1,2-dianilinoethane 1.74 PC 3-methyl-2-butanol 3.66 C 1,2-naphthoquinone 1.07 PC 3-methylcholanthrene 10.30 U 1,3,5-trichlorobenzene 6.67 C 3-pentanol 5.00 C 1,3-butadiene 8.12 C 4-ethylpyridine 2.87 C 1,3-dichloroacetone 5.65 C 4-nitrosophenol 3.98 C 1,3-diphenylguanidine 1.21 PC 4-phenylsemicarbazide -0.17 U 1,4-naphthoquinone 4.37 C 5-bromouracil -0.42 U 16-epiestriol 3.19 C 5-diazouracil 3.71 C 1-butene 5.06 C 5-methoxytryptamine 1.99 PC 1-dodecanol 1.87 PC 5-nitrobarbituric 1.51 PC 1-fluoro-2,4-dinitrobenzene 0.19 U 6-azauridine -3.15 I 1h-1,2,4-triazole 3.75 C 8-azaguanine 0.30 U 1-naphthoic,acid 0.21 U 9,10-dibromoanthracene 8.02 C 1-naphthol 1.65 PC Acebutolol -5.12 I 1-nitropropane 4.63 C Acecainide -3.39 I 1-nitroso-2-naphthol 1.95 PC Aceclofenac -3.27 I 1-pentene 4.41 C Acedapsone -0.13 U 1-phenyl-3-pyrazolidinone -0.91 U Acediasulfone -1.23 PI 2,4,6-tribromophenol 2.77 C Aceglatone