1 APPENDICES Appendix 1. Inhalers Included in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Medicaid Policy Change
MEDICAID POLICY CHANGE IMMINENT PERIL JUSTIFICATION September 25, 2019 ADVAIR: POLICY CHANGE: LDH is changing the preferred drug list to switch the diskus inhaled powder from preferred to non-preferred and adding the HFA inhaler to the preferred list instead. JUSTIFICATION: This product is used to control symptoms and prevent complications caused by asthma or chronic obstructive pulmonary disease. This change is necessary to make an easier delivery device available for recipients to aid with treatment. Without preferred status, recipients would be required to obtain prior authorization which could delay necessary treatment. This change is needed by 10/1/19 due to the coming seasonal change in weather, including influenza and allergy season, that can significantly exacerbate chronic lung diseases, and so this presents an imminent peril to public health. EFFECTIVE DATE: October 1, 2019 LA Medicaid Preferred Drug List (PDL)/Non-Preferred Drug List (NPDL) Effective Date: July 15October 1, 2019 AG – Authorized Generic DR – Concurrent Prescriptions Must Be Written by Same Prescriber PU – Prior Use of Other Medication is Required AL – Age Limits DS – Maximum Days’ Supply Allowed QL – Quantity Limits BH – Behavioral Health Clinical Authorization Required for Children Younger Than 6 DT – Duration of Therapy Limit RX – Specific Prescription Requirements Years Old BY – Diagnosis Codes Bypass Some Requirements DX – Diagnosis Code Requirements TD – Therapeutic Duplication UN – Drug Use Not Warranted (Needs Appropriate CL – More Detailed Clinical Information -

Chronic Obstructive Lung Disease
Chronic Obstructive Lung Disease Amita Vasoya, DO FACOI FCCP FAASM Christiana Care Pulmonary Associates Clinical Assistant Professor of Medicine Sidney Kimmel Medical College of Thomas Jefferson University Rowan University School of Osteopathic Medicine ACOI Board Review 2019 Disclosures No Disclosures Obstructive Lung Diseases COPD Chronic ◦ Chronic Bronchitis Bronchitis Emphysema ◦ Emphysema Asthma Other ◦ Bronchiectasis Asthma ◦ Bronchiolitis ◦ Cystic Fibrosis ◦ Alpha 1 anti-trypsin deficiency Inter-relationship: Inflammation and Bronchial Hyperreactivity ATS GOLD CHEST 2002; 121: 121S-126S COPD THIRD leading cause of death worldwide It is the only leading cause of death whose prevalence is increasing! http://www.who.int/mediacentre/factsheets COPD Risk Factors Cigarette smoking Occupational exposures ◦ Silica, formaldehyde, toluene, nickel, cadmium, cotton, dust, etc Air pollution Biomass fuel Hyperresponsive airway Asthma Genetic factors Pathogenesis of COPD ATS Pulmonary Board Review 2015 Inflammatory Mediators: COPD ATS Pulmonary Board Review 2015 INFLAMMATION Small Airway Disease Parenchyma destruction Airway inflammation Loss of alveolar attachments Airway remodeling Decreased elastic recoil AIRFLOW LIMITATION ATS Pulmonary Board Review 2015 COPD Phenotypes Non-exacerbator Exacerbator with emphysema Exacerbator with chronic bronchitis Frequent exacerbator Alpha 1 Antitrypsin deficiency ACOS BCOS www.eclipse-copd.com, Lange P. Int J COPD 2016. 11: 3-12 Hurst JR. NEJM 2010. 363: 1128-38 Morphologic Types of -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Effects of Glycopyrronium on Lung Hyperinflation in COPD Patients Claudio M Sanguinetti1,2
Sanguinetti Multidisciplinary Respiratory Medicine 2014, 9:19 http://www.mrmjournal.com/content/9/1/19 REVIEW Open Access The lungs need to be deflated: effects of glycopyrronium on lung hyperinflation in COPD patients Claudio M Sanguinetti1,2 Abstract Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation caused by bronchial alterations, small airways disease and parenchymal destruction. In patients with COPD the structural and functional lung alterations can progress more or less rapidly from the initial small airways disease to an overt COPD where a severe expiratory flow limitation takes place. In these conditions, lung hyperinflation develops characterized by increase in functional residual capacity (FRC) and decrease in inspiratory capacity (IC). Thus, IC is an easy and reliable index to monitor lung hyperinflation and to assess the efficacy of bronchodilator drugs. When FRC increases, tidal volume (VT) is located in a more flatted upper part of the P –V curve of the respiratory system and respiratory muscles must sustain a greater elastic workload. Furthermore, due to inadequate time for expiration, there is a positive alveolar pressure at the end of expiration (PEEPi). This represents a further elastic workload for the inspiratory muscles. This impairment of ventilatory mechanics generates dyspnea that in most severely compromised patients occurs also for small efforts causing activity limitation and worst health-related quality of life (HRQoL). Due to these respiratory alterations, bronchodilators are the cornerstone of the long-term treatment of COPD in order to decrease airways resistances, lung hyperinflation and exacerbation rate, and improve patient’s symptoms, exercise tolerance and health status. -

Effects of Replacing Oxitropium with Tiotropium on Pulmonary Function in Patients with COPD: a Randomized Study
View metadata, citation and similar papers at core.ac.uk ARTICLE IN PRESS brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2007) 101, 476–480 Effects of replacing oxitropium with tiotropium on pulmonary function in patients with COPD: A randomized study Cristoforo IncorvaiaÃ, Gian Galeazzo Riario-Sforza, Chiara Pravettoni, Natale Dugnani, Fulvia Paterniti, Laura Pessina, Mario Fumagalli Unit of Pulmonary Rehabilitation, ICP Hospital, via Bignami 1, Viale Molise 69, Milan, Italy Received 3 February 2006; accepted 6 July 2006 KEYWORDS Summary COPD; Background: Inhaled bronchodilators are first line drugs in the treatment of chronic Pulmonary function; obstructive pulmonary disease (COPD). Tiotropium bromide is a recently introduced long- Drug treatment; acting anticholinergic agent able to reduce dyspnoea and COPD exacerbations and to Anticholinergics; improve pulmonary function and quality of life. We designed a study to compare the short- Tiotropium; term efficacy of tiotropium bromide with that of oxitropium bromide in improving Oxitropium pulmonary function in patients with COPD. Methods: Eighty patients were randomized either to continue oxitropium 800 mcg/day or to receive tiotropium 18 mcg/day. Seventy-six (39 in the tiotropium and 37 in the oxitropium group) completed the study. Plethysmography was performed at baseline and after 72 h in all patients. The changes in functional parameters in the two groups were compared by the Mann–Whitney U-test. Results: There were no differences between the two groups regarding age (72.5 vs. 74.2 years), male/female ratio (25/14 vs. 23/14) and pulmonary function at baseline. The changes in spirometric parameters were significantly greater in tiotropium- than in oxitropium-treated patients: mean forced expiratory volume in 1 s (FEV1) increased significantly by 15% vs. -

Arformoterol Tartrate) Inhalation Solution 3 15 Mcg*/2 Ml 4 *Potency Expressed As Arformoterol 5 6 for Oral Inhalation Only 7 8 WARNING: ASTHMA RELATED DEATH
® 1 BROVANA 2 (arformoterol tartrate) Inhalation Solution 3 15 mcg*/2 mL 4 *potency expressed as arformoterol 5 6 For oral inhalation only 7 8 WARNING: ASTHMA RELATED DEATH 9 Long-acting beta2-adrenergic agonists (LABA) increase the risk of asthma-related 10 death. Data from a large placebo-controlled US study that compared the safety of 11 another long-acting beta2-adrenergic agonist (salmeterol) or placebo added to usual 12 asthma therapy showed an increase in asthma-related deaths in patients receiving 13 salmeterol. This finding with salmeterol is considered a class effect of LABA, 14 including arformoterol, the active ingredient in BROVANA (see WARNINGS). The 15 safety and efficacy of BROVANA in patients with asthma have not been established. 16 All LABA, including BROVANA, are contraindicated in patients with asthma 17 without use of a long-term asthma control medication (see 18 CONTRAINDICATIONS). 19 20 DESCRIPTION 21 BROVANA (arformoterol tartrate) Inhalation Solution is a sterile, clear, colorless, 22 aqueous solution of the tartrate salt of arformoterol, the (R,R)-enantiomer of formoterol. 23 Arformoterol is a selective beta2-adrenergic bronchodilator. The chemical name for 24 arformoterol tartrate is formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2 25 (4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2R,3R)-2,3 26 dihydroxybutanedioate (1:1 salt), and its established structural formula is as follows: OH H N . HO OH CH3 HO OCH3 HOOC COOH HN H 27 O 28 The molecular weight of arformoterol tartrate is 494.5 g/mol, and its empirical formula ٠C4H6O6 (1:1 salt). -
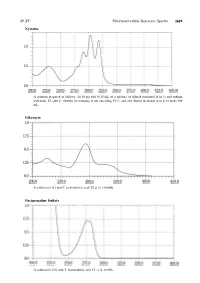
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible