A Case Study of Sex-Linkage in Python Regius(Serpentes: Boidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Skull of the Upper Cretaceous Snake Dinilysia Patagonica Smith-Woodward, 1901, and Its Phylogenetic Position Revisited
Zoological Journal of the Linnean Society, 2012, 164, 194–238. With 24 figures The skull of the Upper Cretaceous snake Dinilysia patagonica Smith-Woodward, 1901, and its phylogenetic position revisited HUSSAM ZAHER1* and CARLOS AGUSTÍN SCANFERLA2 1Museu de Zoologia da Universidade de São Paulo, Avenida Nazaré 481, Ipiranga, 04263-000, São Paulo, SP, Brasil 2Laboratorio de Anatomía Comparada y Evolución de los Vertebrados. Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Av. Angel Gallardo 470 (1405), Buenos Aires, Argentina Received 23 April 2010; revised 5 April 2011; accepted for publication 18 April 2011 The cranial anatomy of Dinilysia patagonica, a terrestrial snake from the Upper Cretaceous of Argentina, is redescribed and illustrated, based on high-resolution X-ray computed tomography and better preparations made on previously known specimens, including the holotype. Previously unreported characters reinforce the intriguing mosaic nature of the skull of Dinilysia, with a suite of plesiomorphic and apomorphic characters with respect to extant snakes. Newly recognized plesiomorphies are the absence of the medial vertical flange of the nasal, lateral position of the prefrontal, lizard-like contact between vomer and palatine, floor of the recessus scalae tympani formed by the basioccipital, posterolateral corners of the basisphenoid strongly ventrolaterally projected, and absence of a medial parietal pillar separating the telencephalon and mesencephalon, amongst others. We also reinterpreted the structures forming the otic region of Dinilysia, confirming the presence of a crista circumfenes- tralis, which represents an important derived ophidian synapomorphy. Both plesiomorphic and apomorphic traits of Dinilysia are treated in detail and illustrated accordingly. Results of a phylogenetic analysis support a basal position of Dinilysia, as the sister-taxon to all extant snakes. -

Epicrates Maurus (Rainbow Boa Or Velvet Mapepire)
UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour Epicrates maurus (Rainbow Boa or Velvet Mapepire) Family: Boidae (Boas and Pythons) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Rainbow boa, Epicrates maurus. [http://squamates.blogspot.com/2010/10/declines-in-snake-and-lizard.html, Downloaded 10 November, 2011] . TRAITS. The rainbow boa, also known as the velvet mapepire, is a snake that grows to a maximum length of 4 feet in males and 4.5 to 5 feet in females. The life span of this species of snake is an average of 20 years if held in captivity and 10 years in the wild. Their name, rainbow boa, originated from their appearance because of an iridescent shine emanating from microscopic ridges on their scales that refract light to produce all the colours of the rainbow. These boas are generally brownish red in colour associated with dark marking during their juvenile life, however this coloration becomes subdued as they get older (Underwood 2009). These snakes are mainly nocturnal and also terrestrial, they have a small head with a narrow neck and a thick body (Boos 2001). Boas are considered primitive snakes and this is supported by the presence of two vestigal, hind limbs which appers as spurs on either side of the cloaca (Conrad 2009). ECOLOGY. Rainbow boas occupy a variety of habitats in Trinidad and Tobago, they can be found in dry tropical forest, rainforest clearings or even close to human settlements such as agricultural communities. Like all boas, they are excellent swimmers, however they restrain from being in contact with water as much as possible. -

Quick Reference Guide: Introduced Constrictors in Florida1 Steve A
WEC302 Quick Reference Guide: Introduced Constrictors in Florida1 Steve A. Johnson and Monica E. McGarrity2 Three non-native species of large constrictor snakes are that these were escaped or released pets. View maps of loca- now breeding in Florida, and several others have been tions where each species has been encountered in Florida encountered but have not yet established wild populations. by visiting the EDDMapS Florida invasive species reporting This fact sheet, best viewed as a pdf (http://edis.ifas.ufl.edu/ portal online at http://www.IveGot1.org. Learn more about pdffiles/UW/UW34700.pdf), is a quick reference guide how to scan for, recognize, and report introduced constric- to identification of the constrictors you are most likely to tors by completing the Introduced Reptile Early Detection encounter in Florida. Although many of these snakes are and Documentation training course. Visit http://ufwildlife. not established in the wild, they are common in the pet ifas.ufl.edu/reddy.shtml to learn more and get REDDy! trade, and each has been spotted in the wild—it is likely Pythons Burmese Python (Python bivittatus) Status: established, breeding populations; range expanding Head: dark arrowhead, light center line, dark and light in Florida wedges under eyes Size: up to 12 feet or longer Body: Giraffe-like spots, dark blotches not connected Figure 1. Burmese python. Credits: Head illustration by USGS; body illustration by Monica E. McGarrity, UF 1. This document is WEC302, one of a series of the Department of Wildlife Ecology and Conservation, UF/IFAS Extension. Original publication date November 2010. Revised February 2014 and June 2017. -

A New Species of Hepatozoon (Apicomplexa: Adeleorina) from Python Regius (Serpentes: Pythonidae) and Its Experimental Transmission by a Mosquito Vector
J. Parasitol., 93(?), 2007, pp. 1189–1198 ᭧ American Society of Parasitologists 2007 A NEW SPECIES OF HEPATOZOON (APICOMPLEXA: ADELEORINA) FROM PYTHON REGIUS (SERPENTES: PYTHONIDAE) AND ITS EXPERIMENTAL TRANSMISSION BY A MOSQUITO VECTOR Michal Sloboda, Martin Kamler, Jana Bulantova´*, Jan Voty´pka*†, and David Modry´† Department of Parasitology, University of Veterinary and Pharmaceutical Sciences, Palacke´ho 1-3, 612 42 Brno, Czech Republic. e-mail: [email protected] ABSTRACT: Hepatozoon ayorgbor n. sp. is described from specimens of Python regius imported from Ghana. Gametocytes were found in the peripheral blood of 43 of 55 snakes examined. Localization of gametocytes was mainly inside the erythrocytes; free gametocytes were found in 15 (34.9%) positive specimens. Infections of laboratory-reared Culex quinquefasciatus feeding on infected snakes, as well as experimental infection of juvenile Python regius by ingestion of infected mosquitoes, were performed to complete the life cycle. Similarly, transmission to different snake species (Boa constrictor and Lamprophis fuliginosus) and lizards (Lepidodactylus lugubris) was performed to assess the host specificity. Isolates were compared with Hepatozoon species from sub-Saharan reptiles and described as a new species based on the morphology, phylogenetic analysis, and a complete life cycle. Hemogregarines are the most common intracellular hemo- 3 genera (Telford et al., 2004). Low host specificity of Hepa- parasites found in reptiles. The Hemogregarinidae, Karyolysi- tozoon spp. is supported by experimental transmissions between dae, and Hepatozoidae are distinguished based on the different snakes from different families. Ball (1967) observed experi- developmental patterns in definitive (invertebrate) hosts oper- mental parasitemia with Hepatozoon rarefaciens in the Boa ating as vectors; all 3 families have heteroxenous life cycles constrictor (Boidae); the vector was Culex tarsalis, which had (Telford, 1984). -

Opinion No. 82-811
TO BE PUBLISHED IN THE OFFICIAL REPORTS OFFICE OF THE ATTORNEY GENERAL State of California JOHN K. VAN DE KAMP Attorney General _________________________ : OPINION : No. 82-811 : of : APRI 28, 1983 : JOHN K. VAN DE KAMP : Attorney General : : JOHN T. MURPHY : Deputy Attorney General : : ________________________________________________________________________ THE HONORABLE ROBERT W. NAYLOR, A MEMBER OF THE CALIFORNIA ASSEMBLY, has requested an opinion on the following question: Does "python" as used in Penal Code section 653o to identify an endangered snake include "anaconda"? CONCLUSION As used in Penal Code section 653o to identify an endangered snake, "python" does not include "anaconda." 1 82-811 ANALYSIS Penal Code section 653o, subd. (a), provides as follows: "It is unlawful to import into this state for commercial purposes, to possess with intent to sell, or to sell within the state, the dead body, or any part or product thereof, of any alligator, crocodile, polar bear, leopard, ocelot, tiger, cheetah, jaguar, sable antelope, wolf (Canis lupus), zebra, whale, cobra, python, sea turtle, colobus monkey, kangaroo, vicuna, sea otter, free-roaming feral horse, dolphin or porpoise (Delphinidae), Spanish lynx, or elephant." "Any person who violates any provision of this section is guilty of a misdemeanor and shall be subject to a fine of not less than one thousand dollars ($1,000) and not to exceed five thousand dollars ($5,000) or imprisonment in the county jail for not to exceed six months, or both such fine and imprisonment, for each violation." (Emphasis added.) We are asked whether or not the term "python" in this statute includes "anaconda." Section 653o was enacted in 1970 (Stats. -

Dispelling Python Regius Myths "Gorzula, Stefan, William Owusu Nsiah, and William Oduro
Dispelling Python regius Myths "Gorzula, Stefan, William Owusu Nsiah, and William Oduro. Survey of the status and By Francis Cosquieri management of the Royal Python (Python regius) in Ghana. Secrétariat CITES, 1997." This paper lists several pythons being found in Before we get deep into the “nitty gritty” of the trees, although points out that the species is subject I want to take a moment to say that the very adaptable to the point of being AHH Royal Python sub-group "Not Just a Pet semi-invasive and responds well to Rock" is the best place to start for getting rid of anthropogenic disturbance. the preconceptions surrounding the poor old Royal Python. Zack Tippie and the other admins have done an incredible job of "Luiselli, Luca, and Francesco Maria Angelici. dispelling the myths surrounding Royal Python "Sexual size dimorphism and natural history behaviour and husbandry, and Zack’s care traits are correlated with intersexual dietary guide for this oft-maligned snake (to be found divergence in royal pythons (Python regius) in the third Advancing Herpetological from the rainforests of southeastern Nigeria." Husbandry Quarterly Newsletter) is one of the Italian Journal of Zoology 65.2 (1998): best I have read. 183-185." - In the meantime, here is a post I made some Half of the male pythons encountered over a time ago on AHH itself bringing together some two year period were found on trees, there is a information on the wild habits of this python. large discrepancy between habitat use by All the papers mentioned here can be found in male and female pythons, and by larger and the AHH Files section. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Investigations Into the Presence of Nidoviruses in Pythons Silvia Blahak1, Maria Jenckel2,3, Dirk Höper2, Martin Beer2, Bernd Hoffmann2 and Kore Schlottau2*
Blahak et al. Virology Journal (2020) 17:6 https://doi.org/10.1186/s12985-020-1279-5 RESEARCH Open Access Investigations into the presence of nidoviruses in pythons Silvia Blahak1, Maria Jenckel2,3, Dirk Höper2, Martin Beer2, Bernd Hoffmann2 and Kore Schlottau2* Abstract Background: Pneumonia and stomatitis represent severe and often fatal diseases in different captive snakes. Apart from bacterial infections, paramyxo-, adeno-, reo- and arenaviruses cause these diseases. In 2014, new viruses emerged as the cause of pneumonia in pythons. In a few publications, nidoviruses have been reported in association with pneumonia in ball pythons and a tiger python. The viruses were found using new sequencing methods from the organ tissue of dead animals. Methods: Severe pneumonia and stomatitis resulted in a high mortality rate in a captive breeding collection of green tree pythons. Unbiased deep sequencing lead to the detection of nidoviral sequences. A developed RT-qPCR was used to confirm the metagenome results and to determine the importance of this virus. A total of 1554 different boid snakes, including animals suffering from respiratory diseases as well as healthy controls, were screened for nidoviruses. Furthermore, in addition to two full-length sequences, partial sequences were generated from different snake species. Results: The assembled full-length snake nidovirus genomes share only an overall genome sequence identity of less than 66.9% to other published snake nidoviruses and new partial sequences vary between 99.89 and 79.4%. Highest viral loads were detected in lung samples. The snake nidovirus was not only present in diseased animals, but also in snakes showing no typical clinical signs. -

WILDLIFE TRADE in AMAZON COUNTRIES: an ANALYSIS of TRADE in CITES-LISTED SPECIES Note by the Executive Secretary 1
CBD Distr. GENERAL CBD/SBSTTA/21/INF/8 17 November 2017 ENGLISH ONLY SUBSIDIARY BODY ON SCIENTIFIC, TECHNICAL AND TECHNOLOGICAL ADVICE Twenty-first meeting Montreal, Canada, 11-14 December 2017 Item 4 of the provisional agenda* WILDLIFE TRADE IN AMAZON COUNTRIES: AN ANALYSIS OF TRADE IN CITES-LISTED SPECIES Note by the Executive Secretary 1. The Executive Secretary is circulating herewith, for the information of participants in the twenty-first meeting of the Subsidiary Body on Scientific, Technical and Technological Advice, a report presenting a comprehensive overview of international trade in wildlife species listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in the Amazon countries: Bolivia; Brazil; Colombia; Ecuador; Guyana; Peru; Suriname; and Venezuela. The analysis provides a baseline of information on trade levels and trends in these countries for the 10-year period 2005-2014, in order to inform trade management in the region. It has been produced in close collaboration with national experts, presenting contextual information and insights into the management of wildlife trade in the region. 2. The report is relevant to the work of the Convention on Biological Diversity, in particular with regard to decision XIII/8, paragraph 5(d), in which the Conference of the Parties requests the Executive Secretary, in collaboration with other members of the Collaborative Partnership on Sustainable Wildlife Management, to continue to support efforts by Parties to combat illicit trafficking in wildlife, in line with United Nations General Assembly resolution 69/314 of 30 July 2015, and to enhance institutional capacities on wildlife conservation and law enforcement with relevant law enforcement bodies, such as the International Consortium on Combating Wildlife Crime. -

Ball Python Care Sheet
Ball Python Care Sheet NATURAL HISTORY: The Ball Python (also called the Royal Python) is by far the most common pet python in this part of the world. Ball pythons are native to sub-Saharan West Africa and thrive in extreme heat and humidity. Ball pythons are generally hardy and tend to do well in captivity if all of their basic husbandry needs are met. They are friendly, easy to handle, and a bit shy — making them a favorite amongst many reptile owners. Males range in size from 2-3 feet and females 3-5 feet. A ball python’s lifespan is variable, however, they can live for up to 25-35 years in captivity. DIET: Ball pythons consume rodents exclusively. Ball pythons can be fed mice or rats depending on the age and size of the snake. As a rule of thumb, the size (largest diameter) of the prey item should be approximately the same diameter as the snake’s body. Feeder rodents can be purchased alive or frozen. I recommend feeding frozen-thawed or pre-killed rodents to all pet snakes because live prey items are able to fight back. Keep in mind that rodents have very strong jaws, with sharp teeth, and are capable of inflicting serious wounds on your pet. Therefore, a live rodent should never be left in a snake’s enclosure without direct supervision. Some people prefer to feed snakes in a separate bin or tank (outside of their primary enclosure) which may help prevent accidental bites while reaching into their primary enclosure. Juvenile ball pythons should be fed weekly and adults may be fed bi-weekly or monthly depending on the size of the prey item. -
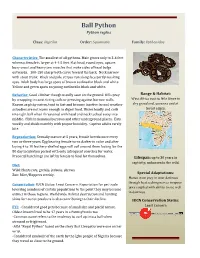
Ball Python Python Regius
Ball Python Python regius Class: Reptilia Order: Squamata Family: Pythonidae Characteristics: The smallest of all pythons. Male grows only to 3-4 feet whereas female is larger at 4-4.5 feet. Flat head, round eyes, square, boxy snout and heavy jaw muscles that make sides of head bulge outwards. 100-150 sharp teeth curve toward the back. Neck narrow with stout trunk. Black and pale stripes run along face partly masking eyes. Adult body has large spots of brown outlined in black and white. Yellow and green spots on young outlined in black and white. Behavior: Good climber though usually seen on the ground. Kills prey Range & Habitat: by wrapping in constricting coils or pressing against burrow walls. West Africa east to Nile River in Known as picky eaters, tend to fast and become inactive in cool weather dry grassland, savanna and at as bodies are not warm enough to digest food. Hisses loudly and coils forest edges. into tight ball when threatened with head and neck tucked away into middle. Hide in mammal burrows and other underground places. Eats weekly and sheds monthly with proper humidity. Captive adults rarely bite. Reproduction: Sexually mature at 5 years, female breeds once every two or three years. Egg bearing female turns darker in color and after laying 4 to 10 leathery-shelled eggs will coil around them fasting for the 80 day incubation period with only infrequent searches for water. Precocial hatchlings are left by female to fend for themselves. Lifespan: up to 30 years in captivity, unknown in the wild. -

THE HUSBANDRY and CAPTIVE PROPAGATION of the SOUTHERN ROCK PYTHON, PYTHON SEBAE NATALENSIS GERALD V HAAGNER Port Elizabeth Snake Park, P.O
BIMCI) I lerpciolopt.)1 Sotici.1 Both:NIL No. 42. 1992/93. THE HUSBANDRY AND CAPTIVE PROPAGATION OF THE SOUTHERN ROCK PYTHON, PYTHON SEBAE NATALENSIS GERALD V HAAGNER Port Elizabeth Snake Park, P.O. Box 13147 Hum wood, 6013. South Africa INTRODUCTION The African Rock Python, Python sebae (Fig. I) is distributed throughout much of sub-Saharan Africa, extending along the Nile valley into Egypt. In southern Africa it is restricted to northern Namibia and Botswana, extending through the northern and eastern Tansvaal, to Natal and the Transkei (Map 1). Pythons were previously found in the eastern Cape Province as far south as Bathurst where it became extinct in 1927 (Broadley, 1983). Branch (1988a) reported on an apparent successful reintroduction of the species into the eastern Cape. Broadley (1984) recently recognised the southern race (Python sebac natalensis) of the African Rock Python as distinct from the typical race from west and central Africa. The recently described Lesser Rock Python (P. saxuloides) from Kenya (Miller & Smith 1979), is treated as a junior synonym of the southern race (Broadley 1983). The African Rock Python is a thick-bodied snake with distinctive markings and size. As with most "giant" snakes. the size is easily over-exaggerated and most recorded lengths have been of the nominate race. Python sebae sebae has been reported to attain a length of 9.8m in the Ivory Coast (Doucet 1963), but full details for this specimen are lacking and cannot now be verified. Arthur Loveridge, whilst collecting in East Africa in 1927, measured a fresh skin of 9.1 metres and even allowing for extensive stretching, it must have been over seven metres long.