Morpholino, Sirna, and S-DNA Compared: Impact of Structure and Mechanism of Action on Off-Target Effects and Sequence Specificity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Multivariate Genome-Wide Association Study of Wing Shape in Drosophila Melanogaster William Pitchers1#A, Jessica Nye2#B, Eladio J
Genetics: Early Online, published on February 21, 2019 as 10.1534/genetics.118.301342 A multivariate genome-wide association study of wing shape in Drosophila melanogaster William Pitchers1#a, Jessica Nye2#b, Eladio J. Márquez2#c, Alycia Kowalski1, Ian Dworkin1*#d, David Houle2* 1 Affiliation: Department of Integrative Biology, Program in Ecology, Evolutionary Biology and Behavior, Michigan State University, East Lansing, Michigan, United States of America 2 Affiliation: Department of Biological Science, Florida State University, Tallahassee, Florida, United States of America #aCurrent Addresss: Microbiological Diagnostic Unit Public Health Laboratory at the University of Melbourne, The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia. #b Current Address: Centre for Research in Agricultural Genomics (CRAG) CSIC-IRTA- UAB-UB, Barcelona, 08193, Spain. #cCurrent Address: The Jackson Laboratory for Genomic Medicine, 10 Discovery Drive, Farmington, Connecticut, United States of America. #dCurrent Address: Department of Biology, McMaster University, Hamilton Ontario, Canada *Authors for correspondence, e-mail: [email protected] & [email protected] Running title: Multivariate GWAS of the fly wing Keywords: Multivariate GWAS, genome-wide association analysis, developmental genetics, phenomics, GP map, Drosophila wing Copyright 2019. Article Summary Understanding the inheritance and evolution of complex traits is an important challenge to geneticists and evolutionary biologists alike. A detailed understanding of how genetic variation affects complex traits is important for the treatment of disease, for our attempts to control the evolution of useful or dangerous organisms, and for understanding and predicting evolution over long time scales. To help understand the evolution and development of the Drosophila melanogaster wing, a model complex trait, we undertook a multivariate genome-wide association study using the Drosophila Genome Reference Panel. -

Split Deoxyribozyme Probe for Efficient Detection of Highly Structured RNA Targets
University of Central Florida STARS Honors Undergraduate Theses UCF Theses and Dissertations 2018 Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets Sheila Raquel Solarez University of Central Florida Part of the Biochemistry Commons, and the Biology Commons Find similar works at: https://stars.library.ucf.edu/honorstheses University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by the UCF Theses and Dissertations at STARS. It has been accepted for inclusion in Honors Undergraduate Theses by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Solarez, Sheila Raquel, "Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets" (2018). Honors Undergraduate Theses. 311. https://stars.library.ucf.edu/honorstheses/311 SPLIT DEOXYRIBOZYME PROBE FOR EFFICIENT DETECTION OF HIGHLY STRUCTURED RNA TARGETS By SHEILA SOLAREZ A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Biological Sciences in the College of Sciences and the Burnett Honors College at the University of Central Florida Orlando, Florida Spring Term, 2018 Thesis Chair: Yulia Gerasimova, PhD ABSTRACT Transfer RNAs (tRNAs) are known for their role as adaptors during translation of the genetic information and as regulators for gene expression; uncharged tRNAs regulate global gene expression in response to changes in amino acid pools in the cell. Aminoacylated tRNAs play a role in non-ribosomal peptide bond formation, post-translational protein labeling, modification of phospholipids in the cell membrane, and antibiotic biosynthesis. [1] tRNAs have a highly stable structure that can present a challenge for their detection using conventional techniques. -

Mrna Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection
International Journal of Molecular Sciences Review mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection 1, 1, 2 1,3, Shuqin Xu y, Kunpeng Yang y, Rose Li and Lu Zhang * 1 State Key Laboratory of Genetic Engineering, Institute of Genetics, School of Life Science, Fudan University, Shanghai 200438, China; [email protected] (S.X.); [email protected] (K.Y.) 2 M.B.B.S., School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; [email protected] 3 Shanghai Engineering Research Center of Industrial Microorganisms, Shanghai 200438, China * Correspondence: [email protected]; Tel.: +86-13524278762 These authors contributed equally to this work. y Received: 30 July 2020; Accepted: 30 August 2020; Published: 9 September 2020 Abstract: Messenger ribonucleic acid (mRNA)-based drugs, notably mRNA vaccines, have been widely proven as a promising treatment strategy in immune therapeutics. The extraordinary advantages associated with mRNA vaccines, including their high efficacy, a relatively low severity of side effects, and low attainment costs, have enabled them to become prevalent in pre-clinical and clinical trials against various infectious diseases and cancers. Recent technological advancements have alleviated some issues that hinder mRNA vaccine development, such as low efficiency that exist in both gene translation and in vivo deliveries. mRNA immunogenicity can also be greatly adjusted as a result of upgraded technologies. In this review, we have summarized details regarding the optimization of mRNA vaccines, and the underlying biological mechanisms of this form of vaccines. Applications of mRNA vaccines in some infectious diseases and cancers are introduced. It also includes our prospections for mRNA vaccine applications in diseases caused by bacterial pathogens, such as tuberculosis. -

Expanding the Genetic Code Lei Wang and Peter G
Reviews P. G. Schultz and L. Wang Protein Science Expanding the Genetic Code Lei Wang and Peter G. Schultz* Keywords: amino acids · genetic code · protein chemistry Angewandte Chemie 34 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/anie.200460627 Angew. Chem. Int. Ed. 2005, 44,34–66 Angewandte Protein Science Chemie Although chemists can synthesize virtually any small organic molecule, our From the Contents ability to rationally manipulate the structures of proteins is quite limited, despite their involvement in virtually every life process. For most proteins, 1. Introduction 35 modifications are largely restricted to substitutions among the common 20 2. Chemical Approaches 35 amino acids. Herein we describe recent advances that make it possible to add new building blocks to the genetic codes of both prokaryotic and 3. In Vitro Biosynthetic eukaryotic organisms. Over 30 novel amino acids have been genetically Approaches to Protein encoded in response to unique triplet and quadruplet codons including Mutagenesis 39 fluorescent, photoreactive, and redox-active amino acids, glycosylated 4. In Vivo Protein amino acids, and amino acids with keto, azido, acetylenic, and heavy-atom- Mutagenesis 43 containing side chains. By removing the limitations imposed by the existing 20 amino acid code, it should be possible to generate proteins and perhaps 5. An Expanded Code 46 entire organisms with new or enhanced properties. 6. Outlook 61 1. Introduction The genetic codes of all known organisms specify the same functional roles to amino acid residues in proteins. Selectivity 20 amino acid building blocks. These building blocks contain a depends on the number and reactivity (dependent on both limited number of functional groups including carboxylic steric and electronic factors) of a particular amino acid side acids and amides, a thiol and thiol ether, alcohols, basic chain. -
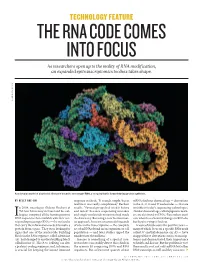
THE RNA CODE COMES INTO FOCUS As Researchers Open up to the Reality of RNA Modification, an Expanded Epitranscriptomics Toolbox Takes Shape
TECHNOLOGY FEATURE THE RNA CODE COMES INTO FOCUS As researchers open up to the reality of RNA modification, an expanded epitranscriptomics toolbox takes shape. LAGUNA DESIGN/SPL LAGUNA A molecular model of a bacterial ribosome bound to messenger RNA, a complex that is formed during protein synthesis. BY KELLY RAE CHI response in check. “It sounds simple, but in mRNAs harbour chemical tags — decorations real life it was really complicated,” Rechavi to the A, C, G and U nucleotides — that are n 2004, oncologist Gideon Rechavi at recalls. “Several groups had tried it before invisible to today’s sequencing technologies. Tel Aviv University in Israel and his col- and failed” because sequencing mistakes (Similar chemical tags, called epigenetic mark- leagues compared all the human genomic and single-nucleotide mutations had made ers, are also found on DNA.) Researchers aren’t IDNA sequences then available with their cor- the data noisy. But using a new bioinformat- sure what these chemical changes in RNA do, responding messenger RNAs — the molecules ics approach, his team uncovered thousands but they’re trying to find out. that carry the information needed to make a of sites in the transcriptome — the complete A wave of studies over the past five years — protein from a gene. They were looking for set of mRNAs found in an organism or cell many of which focus on a specific RNA mark signs that one of the nucleotide building population — and later studies upped the called N6-methyladenosine (m6A) — have blocks in the RNA sequence, called adenosine number into the millions1. -

Replication and Kinetic Trapping of Nucleic Acids in Alternative Environments
REPLICATION AND KINETIC TRAPPING OF NUCLEIC ACIDS IN ALTERNATIVE ENVIRONMENTS A Dissertation Presented to The Academic Faculty by Adriana Lozoya Colinas In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry and Biochemistry Georgia Institute of Technology December 2020 COPYRIGHT © 2020 BY ADRIANA LOZOYA COLINAS REPLICATION AND KINETIC TRAPPING OF NUCLEIC ACIDS IN ALTERNATIVE ENVIRONMENTS Approved by: Dr. Nicholas V. Hud, Advisor Dr. Amanda Stockton School of Chemistry and Biochemistry School of Chemistry and Biochemistry Georgia Institute of Technology Georgia Institute of Technology Dr. Martha A. Grover Dr. Adegboyega (Yomi) Oyelere School of Chemical & Biomolecular School of Chemistry and Biochemistry Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. Loren Williams School of Chemistry and Biochemistry Georgia Institute of Technology Date Approved: October 16, 2020 ACKNOWLEDGEMENTS I would like to thank my mom and dad for all their support and setting up an example for me to follow. I really appreciate everything you have done to encourage me to succeed and follow my dreams. I want to thank Mario for always supporting me. I know it hasn’t always been easy being far away, thank you for being patient and supportive with me. Thank you for the time and adventures we lived together. To all my Latino family at Georgia Tech, thank you for making me feel closer to home. We spent a lot of time together, learned a lot from each other and shared our culture, all of which have made my PhD experience more enjoyable. I would also like to acknowledge my advisor, Nick Hud, for being supportive and sharing his passion for science with me. -

Mrna, Rrna and Trna Types of RNA: Mrna, Rrna and Trna
Types of RNA: mRNA, rRNA and tRNA Types of RNA: mRNA, rRNA and tRNA By Susha Cheriyedath, M.Sc. Reviewed by Michael Greenwood, M.Sc. RNA or ribonucleic acid is a polymer of nucleotides that is made up of a ribose sugar, a phosphate, and bases such as adenine, guanine, cytosine, and uracil. It plays a crucial role in gene expression by acting as the intermediate between the genetic information encoded by DNA and proteins. Designua | Shutterstock RNA has a structure very similar to that of DNA. The key difference in RNA structure is that the ribose sugar in RNA possesses a hydroxyl (OH) group that is absent in DNA. Types of RNA In both prokaryotes and eukaryotes, there are three main types of RNA – messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). These 3 types of RNA are discussed below. P Saved from URL: https://www.news-medical.net/life-sciences/-Types-of-RNA-mRNA-rRNA-and-tRNA.aspx 1/5 Types of RNA: mRNA, rRNA and tRNA Messenger RNA (mRNA) mRNA accounts for just 5% of the total RNA in the cell. mRNA is the most heterogeneous of the 3 types of RNA in terms of both base sequence and size. It carries complimentary genetic code copied, from DNA during transcription, in the form of triplets of nucleotides called codons. Each codon specifies a particular amino acid, though one amino acid may be coded for by many different codons. Although there are 64 possible codons or triplet bases in the genetic code, only 20 of them represent amino acids. -

Guide for Morpholino Users: Toward Therapeutics
Open Access Journal of Drug Discovery, Development and Delivery Special Article - Antisense Drug Research and Development Guide for Morpholino Users: Toward Therapeutics Moulton JD* Gene Tools, LLC, USA Abstract *Corresponding author: Moulton JD, Gene Tools, Morpholino oligos are uncharged molecules for blocking sites on RNA. They LLC, 1001 Summerton Way, Philomath, Oregon 97370, are specific, soluble, non-toxic, stable, and effective antisense reagents suitable USA for development as therapeutics and currently in clinical trials. They are very versatile, targeting a wide range of RNA targets for outcomes such as blocking Received: January 28, 2016; Accepted: April 29, 2016; translation, modifying splicing of pre-mRNA, inhibiting miRNA maturation and Published: May 03, 2016 activity, as well as less common biological targets and diagnostic applications. Solutions have been developed for delivery into a range of cultured cells, embryos and adult animals; with development of a non-toxic and effective system for systemic delivery, Morpholinos have potential for broad therapeutic development targeting pathogens and genetic disorders. Keywords: Splicing; Duchenne muscular dystrophy; Phosphorodiamidate morpholino oligos; Internal ribosome entry site; Nonsense-mediated decay Morpholinos: Research Applications, the transcript from miRNA regulation; Therapeutic Promise • Block regulatory proteins from binding to RNA, shifting Morpholino oligos bind to complementary sequences of RNA alternative splicing; and get in the way of processes. Morpholino oligos are commonly • Block association of RNAs with cytoskeletal motor protein used to prevent a particular protein from being made in an organism complexes, preventing RNA translocation; or cell culture. Morpholinos are not the only tool used for this: a protein’s synthesis can be inhibited by altering DNA to make a null • Inhibit poly-A tailing of pre-mRNA; mutant (called a gene knockout) or by interrupting processes on RNA • Trigger frame shifts at slippery sequences; (called a gene knockdown). -

Transcription Study Guide This Study Guide Is a Written Version of the Material You Have Seen Presented in the Transcription Unit
Transcription Study Guide This study guide is a written version of the material you have seen presented in the transcription unit. The cell’s DNA contains the instructions for carrying out the work of the cell. These instructions are used by the cell’s protein-making machinery to create proteins. If the cell’s DNA were directly read by the protein-making machinery, however, it could be damaged and the process would be slow and cumbersome. The cell avoids this problem by copying genetic information from its DNA into an intermediate called messenger RNA (mRNA). It is this mRNA that is read by the cell’s protein-making machinery. This process is called transcription. Components In this section you will be introduced to the components involved in the process of RNA synthesis, called transcription. This process requires an enzyme that uses many nucleotide bases to copy the instructions present in DNA into an intermediate messenger RNA molecule. RNA What is RNA? · Like DNA, RNA is a polymer made up of nucleotides. · Unlike DNA, which is composed of two strands of nucleotides twisted together, RNA is single-stranded. It can also sometimes fold into complex three-dimensional structures. · RNA contains the same nucleotides as DNA, with the substitution of uraciluridine (U) for thymidine (T). · RNA is chemically different from DNA so that the cell can easily tell the two apart. · In this animation, you will see one type of RNA, messenger RNA, being put together. · There are three types of RNA: mRNA, which you will read more about; tRNA, which is used in the translation process, and rRNA, which acts as a structural element in the ribosome (a translation component). -

Isolation of RNA with Properties of Messenger RNA from Cerebral Polyribosomes* Claire E
Proceedings of the National Academy of Sciences Vol. 67, No. 2, pp. 644-651, October 1970 Isolation of RNA with Properties of Messenger RNA from Cerebral Polyribosomes* Claire E. Zomzelyt, Sidney Roberts, and Susan Peache DEPARTMENT OF BIOLOGICAL CHEMISTRY, SCHOOL OF MEDICINE, AND THE BRAIN RESEARCH INSTI- TUTE, UNIVERSITY OF CALIFORNIA CENTER FOR THE HEALTH SCIENCES, LOS ANGELES, CALIFORNIA 90024 Communicated by H. W. Magoun, July 24, 1970 Abstract. RNA which dissociated from purified cerebral polyribosomes of adult rats in the presence of EDTA was isolated by fractionation in a dis- continuous sucrose gradient. The yield was 2% of the total polyribosomal RNA. The base composition resembled the complementary values for rat DNA and was very different from base compositions of ribosomal RNA and transfer RNA. This RNA fraction contained a large proportion of molecules which were rapidly labeled in vivo and hybridized to homologous DNA. The polyribosomal RNA preparation also exhibited high template activity in a cerebral cell-free system which had previously been stripped of the capacity to incorporate amino acids in the absence of added messenger RNA (mRNA). Sedimentation analysis revealed only two peaks, with coefficients of approximately 8 S and 16 S. The data indicate that RNA with the properties of mRNA can be selectively isolated from cerebral polyribosomes under mild conditions which avoid degradation. Numerous studies support the concept that alterations in RNA and protein synthesis are associated with information transfer and storage in the central nervous system. Activation of the synthesis of specific proteins is suggested by the reports that new RNA with altered base composition, presumably mRNA, is formed during the acquisition phase of learning. -

Long Non-Coding Rnas Are Largely Dispensable for Zebrafish Embryogenesis, Viability and Fertility
bioRxiv preprint doi: https://doi.org/10.1101/374702; this version posted July 23, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Long non-coding RNAs are largely dispensable for zebrafish embryogenesis, viability and fertility Mehdi Goudarzi1, Kathryn Berg1, Lindsey M. Pieper1, and Alexander F. Schier1,2,3,4,5 1Department oF Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts, USA. 2Center For Brain Science, Harvard University, Cambridge, MA 02138, USA., 3FAS Center For Systems Biology, Harvard University, Cambridge, MA 02138, USA., 4Allen Discovery Center For Cell Lineage Tracing, University oF Washington, Seattle, 5 WA 98195, USA., Biozentrum, University oF Basel, Switzerland. Correspondence should be addressed to M.G. ([email protected]) or A.F.S. ([email protected]). Hundreds oF long non-coding RNAs (lncRNAs) have been identiFied as potential regulators oF gene expression, but their Functions remain largely unknown. To study the role oF lncRNAs during vertebrate development, we selected 25 zebraFish lncRNAs based on their conservation, expression proFile or proximity to developmental regulators, and used CRISPR-Cas9 to generate 32 deletion alleles. We observed altered transcription oF neighboring genes in some mutants, but none oF the lncRNAs were required For embryogenesis, viability or Fertility. Even RNAs with previously proposed non-coding Functions (cyrano and squint) and other conserved lncRNAs (gas5 and lnc-setd1ba) were dispensable. In one case (lnc-phox2bb), absence oF putative DNA regulatory-elements, but not of the lncRNA transcript itselF, resulted in abnormal development. -

Targeting Protein Translation, RNA Splicing, and Degradation by Morpholino-Based Conjugates in Plasmodium Falciparum
Targeting protein translation, RNA splicing, and degradation by morpholino-based conjugates in Plasmodium falciparum Aprajita Garga, Donna Wesolowskib, Dulce Alonsob,1, Kirk W. Deitschc, Choukri Ben Mamouna, and Sidney Altmanb,2 aDepartment of Internal Medicine, Yale University School of Medicine, New Haven, CT 06520; bDepartment of Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06520; and cDepartment of Microbiology and Immunology, Weill Medical College of Cornell University, New York, NY 10065 Contributed by Sidney Altman, August 11, 2015 (sent for review May 27, 2015; reviewed by Ron Dzikowski and Rima Mcleod) Identification and genetic validation of new targets from available same targets, MO conjugates have also been used to inhibit RNA genome sequences are critical steps toward the development of splicing and initiation of protein translation (9, 17, 18). new potent and selective antimalarials. However, no methods are To enhance cellular uptake of morpholino oligomers and other currently available for large-scale functional analysis of the Plasmo- drug-like molecules, arginine-rich peptides and polyguanidino dium falciparum genome. Here we present evidence for successful dendrimers have been used (19–23). For morpholino-based anti- use of morpholino oligomers (MO) to mediate degradation of target microbial activity, two types of conjugates have been developed, mRNAs or to inhibit RNA splicing or translation of several genes of PPMOs and vivo morpholino oligomers (VMOs, octa-guanidinium P. falciparum involved in chloroquine transport, apicoplast biogen- dendrimer-conjugated MOs; Materials and Methods). PPMOs are esis, and phospholipid biosynthesis. Consistent with their role in the produced following conjugation of a specific MO to a cell-pen- parasite life cycle, down-regulation of these essential genes resulted etrating, arginine-rich peptide, whereas VMOs are synthesized in inhibition of parasite development.