Beigene Corporate Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BIO-2005-MK-ONLINE (Page 1)
THE SCIENCE. THE BUSINESS. THE WORLD OF BIOPHARMACEUTICALS. 2005 MEDIA PLANNER www.biopharminternational.com THE PRINT-BASED MARKETING Display Advertising BIOPHARM The foundation of any b-to-b marketing program, print advertising allows you to BRAND touch 29,200 BPA-qualified subscribers* with your brand or product message. BioPharm International’s Build your marketing plan around the message portfolio of products allows conveyed in your print ad and utilize other channels you to reach our highly to further reinforce your message. desirable audience through multiple marketing channels. Custom Publishing Work with us to write and design a custom white paper or article Studies show integrated published as an advertorial in the pages of BioPharm. marketing is the key to successfully delivering your Insert A unique way to deliver white papers, brochures or any other collateral message to today’s buyer. material to our readers. Reinforce your message to the most important audience Polybag in your market—ask your Talk about premium placement! Send your printed piece as a ride-along advertising sales manager and readers will get the message as soon as the issue hits their desk. to design an integrated program Direct Mail utilizing the channels that best Planning a direct mail campaign to reinforce your ad message? meet your marketing objectives. Rent our list to ensure your marketing material reaches your most important audience. Post-It Note A truly unique way to send your message to our readers, Post-It Notes serve as front cover reminders about your brand. Use Post-Its to call out your ads in the issue or run 6 or 12 Post-It Notes for a truly unique campaign. -

Breaking Eroom's
Breaking Eroom’s Law Michael S. Ringel, Jack W. Scannell, Mathias Baedeker and Ulrik Schulze https://doi.org/10.1038/d41573-020-00059-3 Supplementary Box 1 | Data and analysis Breaking Eroom’s Law The count and value of NMEs relative to R&D spend comes from BCG’s New Therapeutic Drug (NTD) Database, which is also the source of BCG’s annual publication in Nat. Rev. Drug. Discov. showing trends in count and value over time.1 FDA approvals are from FDA’s Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER),2 peak sales estimates are from EvaluatePharma®,3 and R&D spend data are from BCG Value Science, inflation-adjusted using the standard global GDP-based inflator from the Economist Intelligence Unit.4 For additional details on methodology, see Schulze, Baedeker, Chen and Greber.5 Eroom’s Law is linear on a log scale through 2010, with an average increase approximately 12% per annum, or a halving of productivity approximately every seven years. If this holds true even after 2010, we would see a continuation of the linear development of the number of drugs approved per billion US$ R&D spending when using a logarithmic scale. To demonstrate that the deviation from regression after 2010 is statistically relevant we assumed the following: 1) Eroom's law is true up to 2010. 2) Eroom's law is not true between 2010 and 2018. To confirm our assumptions, we used a log-linear regression to describe the number of new molecular entities (NMEs) approved by the US FDA per billion US$ R&D spending from 1950–2010. -

Mirati's Clinical Programs
NASDAQ: MRTX Targeting the genetic and immunological drivers of cancer Corporate Presentation August 2019 1 Safe Harbor Statement Certain statements contained in this presentation, other than statements of fact that are independently verifiable at the date hereof, are "forward-looking" statements, within the meaning of the Private Securities Litigation Reform Act of 1955, that involve significant risks and uncertainties. Forward looking statements can be identified by the use of forward looking words such as “believes,” “expects,” “hopes,” “may,” “will,” “plan,” “intends,” “estimates,” “could,” “should,” “would,” “continue,” “seeks,” “pro forma,” or “anticipates,” or other similar words (including their use in the negative), or by discussions of future matters such as the development of current or future product candidates, timing of potential development activities and milestones, business plans and strategies, possible changes in legislation and other statements that are not historical. Forward-looking statements are based on current expectations of management and on what management believes to be reasonable assumptions based on information currently available to them, and are subject to risks and uncertainties. Such risks and uncertainties may cause actual results to differ materially from those anticipated in the forward-looking statements. Such risks and uncertainties include without limitation potential delays in development timelines, negative clinical trial results, reliance on third parties for development efforts, changes in the competitive landscape, changes in the standard of care, as well as other risks detailed in Mirati's recent filings on Forms 10-K and 10-Q with the U.S. Securities and Exchange Commission. Except as required by law, Mirati undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances, or to reflect the occurrence of unanticipated events. -

List of Section 13F Securities
List of Section 13F Securities 1st Quarter FY 2004 Copyright (c) 2004 American Bankers Association. CUSIP Numbers and descriptions are used with permission by Standard & Poors CUSIP Service Bureau, a division of The McGraw-Hill Companies, Inc. All rights reserved. No redistribution without permission from Standard & Poors CUSIP Service Bureau. Standard & Poors CUSIP Service Bureau does not guarantee the accuracy or completeness of the CUSIP Numbers and standard descriptions included herein and neither the American Bankers Association nor Standard & Poor's CUSIP Service Bureau shall be responsible for any errors, omissions or damages arising out of the use of such information. U.S. Securities and Exchange Commission OFFICIAL LIST OF SECTION 13(f) SECURITIES USER INFORMATION SHEET General This list of “Section 13(f) securities” as defined by Rule 13f-1(c) [17 CFR 240.13f-1(c)] is made available to the public pursuant to Section13 (f) (3) of the Securities Exchange Act of 1934 [15 USC 78m(f) (3)]. It is made available for use in the preparation of reports filed with the Securities and Exhange Commission pursuant to Rule 13f-1 [17 CFR 240.13f-1] under Section 13(f) of the Securities Exchange Act of 1934. An updated list is published on a quarterly basis. This list is current as of March 15, 2004, and may be relied on by institutional investment managers filing Form 13F reports for the calendar quarter ending March 31, 2004. Institutional investment managers should report holdings--number of shares and fair market value--as of the last day of the calendar quarter as required by Section 13(f)(1) and Rule 13f-1 thereunder. -

Promotion and Development Collaborations Between Established Players
Promotion and Development Collaborations Between Established Players Randall B. Sunberg Pharma and Biotech In-Licensing, Co-Development and Co-Promotion Agreements October 21-22, 2002 1 www.morganlewis.com MorganMorgan Lewis:Lewis: LifeLife SciencesSciences FocusFocus • Founded in 1873, today 1,100 lawyers in 13 offices worldwide • 175 Life Sciences professionals, including 90 with advanced degrees ranging from biochemistry to molecular genetics to immunology • Interdisciplinary coordination of transactions, IP, litigation, FDA and antitrust expertise to meet our clients’ strategic objectives • Recently ranked as the 5th leading transactional law firm in the nation by The American Lawyer "Corporate Scorecard” • Named by many Fortune 250 companies as one of their primary law firms • Recognized by corporate counsel for exceptional client service in a survey of Fortune 1000 companies • Featured prominently in the annual National Law Journal survey of “Who Defends Corporate America” 2 MorganMorgan Lewis:Lewis: RecentRecent LifeLife SciencesSciences DealsDeals • Aventis - Genta collaboration for co-development and commercialization of Genasense™ • Enzon - Elan acquisition of Abelcet® product rights and manufacturing assets • Quintiles - Eli Lilly co-promotion and marketing collaboration for Cymbalta™ • Adolor - GlaxoSmithKline development and commercialization collaboration for Alvimopan™ • Arena Pharmaceuticals - Merck drug discovery collaboration • Cephalon - Anesta stock-for-stock public company acquisition • Aventis - Millennium alliance -

Beigene, Ltd. 百濟神州有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 06160)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. BeiGene, Ltd. 百濟神州有限公司 (incorporated in the Cayman Islands with limited liability) (Stock Code: 06160) INSIDE INFORMATION CHINA NMPA APPROVES BRUKINSA® (ZANUBRUTINIB) FOR THE TREATMENT OF PATIENTS WITH RELAPSED OR REFRACTORY WALDENSTRÖM’S MACROGLOBULINEMIA This announcement is issued pursuant to Rule 13.09 of the Rules Governing the Listing of the Securities on The Stock Exchange of Hong Kong Limited and under Part XIVA of the Securities and Futures Ordinance (Cap. 571). On June 18, 2021 (U.S. Eastern Time), BeiGene, Ltd. (“BeiGene” or the “Company”) announced that BRUKINSA® (zanubrutinib) has received conditional approval from the China National Medical Products Administration (NMPA) for the treatment of adult patients with Waldenström’s macroglobulinemia (WM) who have received at least one prior therapy. The supplemental new drug application was previously granted priority review by the Center for Drug Evaluation (CDE) of the NMPA in October 2020. Attached hereto as Schedule 1 is the full text of the press release issued by the Company on June 18, 2021 (U.S. Eastern Time) announcing the above-described business updates. 1 Forward-Looking Statements This announcement contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the potential clinical benefits and advantages of BRUKINSA compared to other BTK inhibitors; BeiGene’s plans for the advancement, and anticipated clinical development, regulatory milestones and commercialization of BRUKINSA; and BeiGene’s plans, commitments, aspirations, and goals under the headings “BeiGene Oncology” and “About BeiGene”. -

Hon55 2630.Pdf
ABSTRACT 245 ifosfamide in 2 pts (12%) and other regimens in 3 pts (18%). Pts kinases in biochemical assays and shown favorable PK/PD properties treated with ibrutinib received 560 mg p.o. daily. Intratechal CT was in preclinical studies. In phase 1 testing, high plasma concentrations added in 5 pts (31%) and 7 pts (47%) in standard and ibrutinib cohort. were achieved, resulting in complete and sustained 24-hour BTK inhi- Radiotherapy was delivered to 3 pts, all in the standard cohort, in one bition in blood and lymph nodes in patients (pts) treated at 160 mg case as consolidation and in 2 cases as salvage. With a median follow- twice daily (bid; Tam. Blood 2016;128:642). Here, we present updated up of 10.4 months, the 1-year PFS and OS of the entire study popula- safety and efficacy data from pts with MCL. tion are 24% and 46%. A statistically significant difference in 1-year Methods: This is a global, phase 1 study investigating zanubrutinib in PFS was observed in favor of ibrutinib versus standard CT (49% vs pts with B-cell malignancies with indication-specific expansion cohorts. In the expansion phase, enrolled pts received zanubrutinib 6%, p = 0.044). The difference in 1-year OS in favor of ibrutinib versus 320 mg daily or 160 mg bid (the RP2D). Treatment emergent adverse standard CT did not reach statistical significance (57% vs 37%, events (TEAEs) were summarized according to NCI CTCAE v4.03 and p = 0.097). In the standard cohort only one pt is alive after allogeneic responses were assessed by CT scans as per Lugano Classification transplantation. -

The Bottom 99
The Top 100 June 1, 2004 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. ABFI AMER BUSINESS FINL SVCS INC Financials ABGX ABGENIX INC Health Care ADLR ADOLOR CORP Health Care AGEN ANTIGENICS INC/DEL Health Care AGI ALLIANCE GAMING CORP Consumer Cyclicals AKLM ACCLAIM ENMNT INC Technology ALKS ALKERMES INC Health Care ALXN ALEXION PHARMACEUTICALS INC Health Care AMLN AMYLIN PHARMACEUTICALS INC Health Care APHT APHTON CORP Health Care ARNA ARENA PHARMACEUTICALS INC Health Care BIOV BIOVERIS CORP Health Care BMRN BIOMARIN PHARMACEUTICAL INC Health Care BVSN BROADVISION INC Technology CBRX COLUMBIA LABORATORIES INC Health Care CCRD CONCORD COMMUNICATIONS INC Technology CDE COEUR D'ALENE MINES CORP Basic Materials CFFN CAPITOL FEDERAL FINANCIAL Financials COMS 3COM CORP Technology CPN CALPINE CORP Utilities CRAY CRAY INC Technology CRGN CURAGEN CORP Health Care CRXA CORIXA CORP Health Care CYBX CYBERONICS INC Health Care DAL DELTA AIR LINES INC Capital Goods DEPO DEPOMED INC Health Care DMX I-TRAX INC Health Care DRXR DREXLER TECHNOLOGY CORP Technology DSCO DISCOVERY LABORATORIES INC Health Care DV DEVRY INC Capital Goods ELGX ENDOLOGIX INC Health Care EPIX EPIX MEDICAL INC Health Care EXEL EXELIXIS INC Health Care EXLT EXULT INC Capital Goods FCP FALCON PRODUCTS INC Capital Goods FMKT FREEMARKETS INC Technology FXEN FX ENERGY INC Energy GBTVK GRANITE BROADCASTING Consumer Cyclicals GENE OSCIENT PHARMACEUTICALS CORP Health Care GGNS GENUS INC Technology GLFD GUILFORD PHARMACEUTICAL INC Health Care GMRK GULFMARK OFFSHORE -

Abstract EP783 MARGINAL ZONE LYMPHOMA (MAGNOLIA STUDY)
PHASE 2 STUDY OF ZANUBRUTINIB IN PATIENTS WITH RELAPSED/REFRACTORY Abstract EP783 MARGINAL ZONE LYMPHOMA (MAGNOLIA STUDY) Stephen Opat,1,2 Alessandra Tedeschi,3 Kim Linton,4 Pamela McKay,5 Bei Hu,6 Henry Chan,7 Jie Jin,8 Magdalena Sobieraj-Teague,9 Pier Luigi Zinzani,10 Morton Coleman,11 Peter Browett,12 Xiaoyan Ke,13 Mingyuan Sun,14 Robert Marcus,15 Craig Portell,16 Catherine Thieblemont,17 Kirit Ardeshna,18,19 Fontanet Bijou,20 Patricia Walker,21 Eliza Hawkes,22-24 Sally Mapp,25 Shir-Jing Ho,26 Melannie Co,27 Xiaotong Li,27 Wenxiao Zhou,27 Massimo Cappellini,27 Chris Tankersley,27 Jane Huang,27 and Judith Trotman28 1Monash Health, Clayton, Victoria, Australia; 2Clinical Haematology Unit Monash University, Clayton, Victoria, Australia; 3ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 4The Christie, Manchester, UK; 5Beatson West of Scotland Cancer Centre, Glasgow, UK; 6Levine Cancer Institute/Atrium Health, Charlotte, NC, USA; 7North Shore Hospital, Auckland, New Zealand; 8The First Affiliated Hospital, Zhejiang University, Hangzhou, China; 9Flinders Medical Centre, Bedford Park, Australia; 10Institute of Hematology “Seràgnoli” University of Bologna, Bologna, Italy; 11Clinical Research Alliance, Lake Success, NY, USA; 12Auckland City Hospital, Grafton, New Zealand; 13Peking University Third Hospital, Beijing, China; 14Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China; 15Sarah Cannon Research Institute UK, London, UK; 16University of Virginia -

Beigene, Ltd. (Incorporated in the Cayman Islands with Limited Liability and Trading As “百濟神州”Or“百濟神州有限公司”) (Stock Code: 06160)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. BeiGene, Ltd. (incorporated in the Cayman Islands with limited liability and trading as “百濟神州”or“百濟神州有限公司”) (Stock Code: 06160) BUSINESS UPDATE BEIGENE INITIATES GLOBAL HEAD-TO-HEAD PHASE 3 CLINICAL TRIAL OF ZANUBRUTINIB IN PATIENTS WITH RELAPSED/REFRACTORY CHRONIC LYMPHOCYTIC LEUKEMIA OR SMALL LYMPHOCYTIC LYMPHOMA On November 7, 2018, BeiGene, Ltd. (“BeiGene” or the “Company”), a commercial-stage biopharmaceutical company focused on developing and commercializing innovative molecularly-targeted and immuno-oncology drugs for the treatment of cancer, announced that the first patient was dosed in a global Phase 3 clinical trial of its investigational BTK inhibitor zanubrutinib compared with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). “We continue to be encouraged by data on zanubrutinib in various B-cell malignancies and are excited to further expand the development program for zanubrutinib in CLL and SLL with this Phase 3 trial, which represents the second Phase 3 study directly comparing zanubrutinib to ibrutinib,” said Jane Huang, M.D., Chief Medical Officer, Hematology, at BeiGene. The global Phase 3 open-label trial is expected to enroll approximately 400 patients with relapsed/refractory CLL or SLL across approximately 150 study centers in the U.S., China, Europe, Australia and New Zealand. -
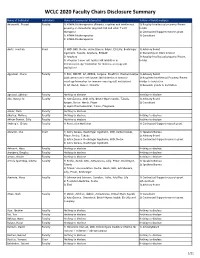
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

Pierre Cremieux October 2020 CV (English)
PIERRE-YVES CREMIEUX, PH.D. President Office: 617 425 8135 111 Huntington Avenue Fax: 617 425 8001 14th Floor [email protected] Boston, MA 02199 Pierre-Yves Cremieux, President of Analysis Group, has a broad range of expertise in health economics, antitrust, statistics, and labor economics. He has consulted to numerous clients in the United States and Canada and testified in bench and jury trials, arbitrations, and administrative proceedings. Dr. Cremieux has served as an expert and supported other experts in both litigation and non-litigation matters on antitrust issues; general commercial claims; contractual disputes; and a number of labor- related matters in a variety of industries, including high tech, pharmaceuticals, biotech, financial products, consumer products, and commodities. He has assessed the evaluation of damages on a class-wide basis in some of the largest class action matters in recent years. His scientific research in antitrust economics, class certification, health economics, and statistics has been published in numerous peer-reviewed journals, including the George Mason Law Review, the American Bar Association Economics Committee Newsletter, The Review of Economics and Statistics, the Journal of Health Economics, Industrial and Labor Relations Review, the Journal of Clinical Oncology, and The American Journal of Managed Care. Dr. Cremieux’s research has been cited in leading media outlets including The Wall Street Journal and Forbes. Dr. Cremieux has frequently presented at leading legal, health care, and economics seminars on topics such as antitrust, class certification, health economics, and statistics, in both the United States and Canada. He has also been invited to teach courses on economics, statistics, health care, and antitrust at various schools including McGill University, Boston University, Harvard Medical School, and the Yale School of Management.