Mirati's Clinical Programs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Immune Checkpoint Inhibitor Combinations Current Efforts And
Drug Resistance Updates 45 (2019) 13–29 Contents lists available at ScienceDirect Drug Resistance Updates journal homepage: www.elsevier.com/locate/drup Immune checkpoint inhibitor combinations: Current efforts and important aspects for success T ⁎ Edo Kon, Itai Benhar Department of Molecular Microbiology and Biotechnology, School of Molecular Cell Biology and Biotechnology, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv 69978, Israel ARTICLE INFO ABSTRACT Keywords: Immune checkpoint inhibitors (ICI) have emerged as a remarkable treatment option for diverse cancer types. Resistance Currently, ICIs are approved for an expanding array of cancer indications. However, the majority of patients still Immunotherapy do not demonstrate a durable long-term response following ICI therapy. In addition, many patients receiving ICI Clinic therapy develop immune-related adverse events (irAEs) affecting a wide variety of organs. To increase the CAR-T percentage of patients who benefit from ICI therapy and to reduce the occurrence of irAEs, there is an ongoing Radiation therapy effort to combine current ICIs with novel checkpoints inhibitors or other therapeutic approaches to achieve a Chemotherapy ff RNA cancer vaccines synergistic e ect which is larger than the sum of its parts. Angiogenesis In this review we highlight the essential factors for more effective ICI combinations. We describe how the Tumor microenvironment design of these strategies should be driven by the tumor's immunological context. We analyze current combi- nation strategies and describe how they can be improved to unleash the immune system's full anti-cancer po- tential as well as convert immunologically "cold" tumors into "hot" ones. -

Immune-Checkpoint Blockade Therapy in Lymphoma
International Journal of Molecular Sciences Review Immune-Checkpoint Blockade Therapy in Lymphoma Ayumi Kuzume 1,2, SungGi Chi 1 , Nobuhiko Yamauchi 1 and Yosuke Minami 1,* 1 Department of Hematology, National Cancer Center Hospital East, Kashiwa 277–8577, Japan; [email protected] (A.K.); [email protected] (S.C.); [email protected] (N.Y.) 2 Department of Hematology, Kameda Medical Center, Kamogawa 296–8602, Japan * Correspondence: [email protected]; Tel.: +81-4-7133-1111; Fax: +81-7133-6502 Received: 11 June 2020; Accepted: 28 July 2020; Published: 30 July 2020 Abstract: Tumor cells use immune-checkpoint pathways to evade the host immune system and suppress immune cell function. These cells express programmed cell-death protein 1 ligand 1 (PD-L1)/PD-L2, which bind to the programmed cell-death protein 1 (PD-1) present on cytotoxic T cells, trigger inhibitory signaling, and reduce cytotoxicity and T-cell exhaustion. Immune-checkpoint blockade can inhibit this signal and may serve as an effective therapeutic strategy in patients with solid tumors. Several trials have been conducted on immune-checkpoint inhibitor therapy in patients with malignant lymphoma and their efficacy has been reported. For example, in Hodgkin lymphoma, immune-checkpoint blockade has resulted in response rates of 65% to 75%. However, in non-Hodgkin lymphoma, the response rate to immune-checkpoint blockade was lower. In this review, we evaluate the biology of immune-checkpoint inhibition and the current data on its efficacy in malignant lymphoma, and identify the cases in which the treatment was more effective. -

Beigene, Ltd. 百濟神州有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 06160)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. BeiGene, Ltd. 百濟神州有限公司 (incorporated in the Cayman Islands with limited liability) (Stock Code: 06160) INSIDE INFORMATION CHINA NMPA APPROVES BRUKINSA® (ZANUBRUTINIB) FOR THE TREATMENT OF PATIENTS WITH RELAPSED OR REFRACTORY WALDENSTRÖM’S MACROGLOBULINEMIA This announcement is issued pursuant to Rule 13.09 of the Rules Governing the Listing of the Securities on The Stock Exchange of Hong Kong Limited and under Part XIVA of the Securities and Futures Ordinance (Cap. 571). On June 18, 2021 (U.S. Eastern Time), BeiGene, Ltd. (“BeiGene” or the “Company”) announced that BRUKINSA® (zanubrutinib) has received conditional approval from the China National Medical Products Administration (NMPA) for the treatment of adult patients with Waldenström’s macroglobulinemia (WM) who have received at least one prior therapy. The supplemental new drug application was previously granted priority review by the Center for Drug Evaluation (CDE) of the NMPA in October 2020. Attached hereto as Schedule 1 is the full text of the press release issued by the Company on June 18, 2021 (U.S. Eastern Time) announcing the above-described business updates. 1 Forward-Looking Statements This announcement contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the potential clinical benefits and advantages of BRUKINSA compared to other BTK inhibitors; BeiGene’s plans for the advancement, and anticipated clinical development, regulatory milestones and commercialization of BRUKINSA; and BeiGene’s plans, commitments, aspirations, and goals under the headings “BeiGene Oncology” and “About BeiGene”. -

Clinical Potential of Kinase Inhibitors in Combination with Immune Checkpoint Inhibitors for the Treatment of Solid Tumors
International Journal of Molecular Sciences Review Clinical Potential of Kinase Inhibitors in Combination with Immune Checkpoint Inhibitors for the Treatment of Solid Tumors Ryuhjin Ahn 1 and Josie Ursini-Siegel 2,3,4,5,* 1 Department of Biological Engineering, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; [email protected] 2 Department of Biochemistry, McGill University, Montréal, QC H3G 1Y6, Canada 3 Lady Davis Institute for Medical Research, Jewish General Hospital, Montréal, QC H3T 1E2, Canada 4 Department of Experimental Medicine, McGill University, Montréal, QC H3A 0G4, Canada 5 Department of Oncology, McGill University, 546 Pine Avenue West, Montréal, QC H2W 1S6, Canada * Correspondence: [email protected]; Tel.: +514-340-8222 (ext. 26557); Fax: +514-340-7502 Abstract: Oncogenic kinases contribute to immunosuppression and modulate the tumor microenvi- ronment in solid tumors. Increasing evidence supports the fundamental role of oncogenic kinase signaling networks in coordinating immunosuppressive tumor microenvironments. This has led to numerous studies examining the efficacy of kinase inhibitors in inducing anti-tumor immune responses by increasing tumor immunogenicity. Kinase inhibitors are the second most common FDA-approved group of drugs that are deployed for cancer treatment. With few exceptions, they inevitably lead to intrinsic and/or acquired resistance, particularly in patients with metastatic disease when used as a monotherapy. On the other hand, cancer immunotherapies, including immune checkpoint inhibitors, have revolutionized cancer treatment for malignancies such as melanoma and Citation: Ahn, R.; Ursini-Siegel, J. lung cancer. However, key hurdles remain to successfully incorporate such therapies in the treatment Clinical Potential of Kinase Inhibitors of other solid cancers. -

Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer
cancers Review Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer Antonio Lopez-Beltran 1,*,† , Alessia Cimadamore 2,† , Ana Blanca 3, Francesco Massari 4 , Nuno Vau 5, Marina Scarpelli 2, Liang Cheng 6 and Rodolfo Montironi 2,* 1 Unit of Anatomic Pathology, Department of Morphological Sciences, Cordoba University Medical School, 14004 Cordoba, Spain 2 Pathological Anatomy, School of Medicine, United Hospitals, Polytechnic University of the Marche Region, 60126 Ancona, Italy; [email protected] (A.C.); [email protected] (M.S.) 3 Maimonides Biomedical Research Institute of Cordoba, Department of Urology, University Hospital of Reina Sofia, 14004 Cordoba, Spain; [email protected] 4 Division of Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, 40138 Bologna, Italy; [email protected] 5 Medical Oncology, Champalimaud Clinical Center, 1400-038 Lisbon, Portugal; [email protected] 6 Department of Pathology and Laboratory Medicine, School of Medicine, Indiana University, Indianapolis, IN 46202, USA; [email protected] * Correspondence: [email protected] or [email protected] (A.L.-B.); [email protected] (R.M.); Tel.: +34-9-5721-8992 (A.L.-B.); +39-0-71-596-4830 (R.M.); Fax: +34-9-5721-8229 (A.L.-B.) † These authors contributed equally to the work. Simple Summary: In this review, we examined relevant clinical trial results with immune check- point inhibitors in patients with metastatic urothelial cancer. We also focused on the potential of immunotherapy in the adjuvant and neoadjuvant setting or as part of drug combinations. Finally, we briefly review the current landscape of biomarkers of response to immune checkpoint inhibitors, such as programmed death-ligand 1 (PD-L1) expression, tumor mutation burden, molecular subtypes Citation: Lopez-Beltran, A.; of bladder cancer, and immune-gene expression profiling. -

Hon55 2630.Pdf
ABSTRACT 245 ifosfamide in 2 pts (12%) and other regimens in 3 pts (18%). Pts kinases in biochemical assays and shown favorable PK/PD properties treated with ibrutinib received 560 mg p.o. daily. Intratechal CT was in preclinical studies. In phase 1 testing, high plasma concentrations added in 5 pts (31%) and 7 pts (47%) in standard and ibrutinib cohort. were achieved, resulting in complete and sustained 24-hour BTK inhi- Radiotherapy was delivered to 3 pts, all in the standard cohort, in one bition in blood and lymph nodes in patients (pts) treated at 160 mg case as consolidation and in 2 cases as salvage. With a median follow- twice daily (bid; Tam. Blood 2016;128:642). Here, we present updated up of 10.4 months, the 1-year PFS and OS of the entire study popula- safety and efficacy data from pts with MCL. tion are 24% and 46%. A statistically significant difference in 1-year Methods: This is a global, phase 1 study investigating zanubrutinib in PFS was observed in favor of ibrutinib versus standard CT (49% vs pts with B-cell malignancies with indication-specific expansion cohorts. In the expansion phase, enrolled pts received zanubrutinib 6%, p = 0.044). The difference in 1-year OS in favor of ibrutinib versus 320 mg daily or 160 mg bid (the RP2D). Treatment emergent adverse standard CT did not reach statistical significance (57% vs 37%, events (TEAEs) were summarized according to NCI CTCAE v4.03 and p = 0.097). In the standard cohort only one pt is alive after allogeneic responses were assessed by CT scans as per Lugano Classification transplantation. -

Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: an Overview
pharmaceuticals Review Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: An Overview Diederick J. van Doorn 1 , Robert Bart Takkenberg 1 and Heinz-Josef Klümpen 2,* 1 Department of Gastroenterology and Hepatology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; [email protected] (D.J.v.D.); [email protected] (R.B.T.) 2 Department of Medical Oncology, Amsterdam UMC, Cancer Center Amsterdam, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands * Correspondence: [email protected]; Tel.: +31-20-566-5983 Abstract: Patients with hepatocellular carcinoma (HCC) face a common type of cancer, which is amongst the most deadly types of cancer worldwide. The therapeutic options range from curative resection or ablation to loco regional therapies in palliative setting and last but not least, systemic treatment. The latter group underwent major changes in the last decade and a half. Since the introduction of sorafenib in 2007, many other systemic treatments have been investigated. Most without success. It took more than ten years before lenvatinib could be added as alternative first-line treatment option. Just recently a new form of systemic treatment, immunotherapy, entered the field of therapeutic options in patients with HCC. Immune checkpoint inhibitors are becoming the new standard of care in patients with HCC. Several reviews reported on the latest phase 1/2 studies and discussed the higher response rates and better tolerability when compared to current standard of care therapies. This review will focus on elaborating the working mechanism of these checkpoint inhibitors, give an elaborate update of the therapeutic agents that are currently available or under research, and will give an overview of the latest trials, as well as ongoing and upcoming trials. -

Follow-Up and Management of Checkpoint Inhibitor Related Toxicities in Cancer Patients
Guideline Resource Unit [email protected] Follow-up and Management of Checkpoint Inhibitor Related Toxicities in Cancer Patients Effective Date: July, 2020 Clinical Practice Guideline SUPP-018 – Version 1 www.ahs.ca/guru Table of Contents Background............................................................................................................................................3 Guideline Question.................................................................................................................................4 Search Strategy......................................................................................................................................4 Target Population...................................................................................................................................4 Recommendations General Approaches to Toxicity Management…....................................................................................5 Dermatology Prevention...........................................................................................................................................5 Anticipation..........................................................................................................................................6 Detection.............................................................................................................................................6 Treatment and Monitoring...................................................................................................................7 -

Abstract EP783 MARGINAL ZONE LYMPHOMA (MAGNOLIA STUDY)
PHASE 2 STUDY OF ZANUBRUTINIB IN PATIENTS WITH RELAPSED/REFRACTORY Abstract EP783 MARGINAL ZONE LYMPHOMA (MAGNOLIA STUDY) Stephen Opat,1,2 Alessandra Tedeschi,3 Kim Linton,4 Pamela McKay,5 Bei Hu,6 Henry Chan,7 Jie Jin,8 Magdalena Sobieraj-Teague,9 Pier Luigi Zinzani,10 Morton Coleman,11 Peter Browett,12 Xiaoyan Ke,13 Mingyuan Sun,14 Robert Marcus,15 Craig Portell,16 Catherine Thieblemont,17 Kirit Ardeshna,18,19 Fontanet Bijou,20 Patricia Walker,21 Eliza Hawkes,22-24 Sally Mapp,25 Shir-Jing Ho,26 Melannie Co,27 Xiaotong Li,27 Wenxiao Zhou,27 Massimo Cappellini,27 Chris Tankersley,27 Jane Huang,27 and Judith Trotman28 1Monash Health, Clayton, Victoria, Australia; 2Clinical Haematology Unit Monash University, Clayton, Victoria, Australia; 3ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 4The Christie, Manchester, UK; 5Beatson West of Scotland Cancer Centre, Glasgow, UK; 6Levine Cancer Institute/Atrium Health, Charlotte, NC, USA; 7North Shore Hospital, Auckland, New Zealand; 8The First Affiliated Hospital, Zhejiang University, Hangzhou, China; 9Flinders Medical Centre, Bedford Park, Australia; 10Institute of Hematology “Seràgnoli” University of Bologna, Bologna, Italy; 11Clinical Research Alliance, Lake Success, NY, USA; 12Auckland City Hospital, Grafton, New Zealand; 13Peking University Third Hospital, Beijing, China; 14Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China; 15Sarah Cannon Research Institute UK, London, UK; 16University of Virginia -

Beigene, Ltd. (Incorporated in the Cayman Islands with Limited Liability and Trading As “百濟神州”Or“百濟神州有限公司”) (Stock Code: 06160)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. BeiGene, Ltd. (incorporated in the Cayman Islands with limited liability and trading as “百濟神州”or“百濟神州有限公司”) (Stock Code: 06160) BUSINESS UPDATE BEIGENE INITIATES GLOBAL HEAD-TO-HEAD PHASE 3 CLINICAL TRIAL OF ZANUBRUTINIB IN PATIENTS WITH RELAPSED/REFRACTORY CHRONIC LYMPHOCYTIC LEUKEMIA OR SMALL LYMPHOCYTIC LYMPHOMA On November 7, 2018, BeiGene, Ltd. (“BeiGene” or the “Company”), a commercial-stage biopharmaceutical company focused on developing and commercializing innovative molecularly-targeted and immuno-oncology drugs for the treatment of cancer, announced that the first patient was dosed in a global Phase 3 clinical trial of its investigational BTK inhibitor zanubrutinib compared with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). “We continue to be encouraged by data on zanubrutinib in various B-cell malignancies and are excited to further expand the development program for zanubrutinib in CLL and SLL with this Phase 3 trial, which represents the second Phase 3 study directly comparing zanubrutinib to ibrutinib,” said Jane Huang, M.D., Chief Medical Officer, Hematology, at BeiGene. The global Phase 3 open-label trial is expected to enroll approximately 400 patients with relapsed/refractory CLL or SLL across approximately 150 study centers in the U.S., China, Europe, Australia and New Zealand. -
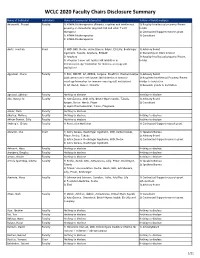
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

Beigene, Ltd. 百濟神州有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 06160)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. BeiGene, Ltd. 百濟神州有限公司 (incorporated in the Cayman Islands with limited liability) (Stock Code: 06160) INSIDE INFORMATION UNAUDITED RESULTS FOR THE THREE MONTHS ENDED MARCH 31, 2021 OF BEIGENE, LTD. AND BUSINESS UPDATES This announcement is issued pursuant to Rule 13.09 of the Rules Governing the Listing of the Securities on The Stock Exchange of Hong Kong Limited and under Part XIVA of the Securities and Futures Ordinance (Cap. 571). BeiGene, Ltd. (the “Company” or “BeiGene”) is pleased to announce its unaudited condensed consolidated financial results for the three months ended March 31, 2021 and business updates. The Company is pleased to announce the unaudited condensed consolidated results of the Company and its subsidiaries for the three months ended March 31, 2021 (the “Q1 Results”) published in accordance with applicable rules of the U.S. Securities and Exchange Commission and business highlights for the first quarter of 2021 and expected milestones for the remainder of 2021 and 2022 (the “Business Updates”). The Q1 Results have been prepared in accordance with U.S. Generally Accepted Accounting Principles, which are different from the International Financial Reporting Standards. Attached hereto as Schedule 1 is the full text of the press release issued by the Company on May 6, 2021 (U.S.