Solvent-Free Organic Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Detection and Determination of Esters
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1958 The etD ection and Determination of Esters. Mohd. Mohsin Qureshi Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Qureshi, Mohd. Mohsin, "The eD tection and Determination of Esters." (1958). LSU Historical Dissertations and Theses. 501. https://digitalcommons.lsu.edu/gradschool_disstheses/501 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. Copright by Mohcl Mohsin Qureshi 1959 THE DETECTION AND DETERMINATION OF ESTERS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Mohd. Mohsin Qureshi M.Sc., Aligarh University, 1944 August, 1958 ACKNOWLEDGMENT The author wishes to express his sincere apprecia tion and gratitude to Dr. Philip W. West under whose guidance this research was carried out. He is grateful to Dr. James G. Traynham for sup plying him with a number of esters and for his many helpful suggestions. The financial support given to him by the Continental Oil Company is gratefully acknowledged. He offers his sincere thanks to Miss Magdalena Usategul for her valuable suggestions and her ungrudging help during the course of this investigation. Dr. Anil K. -

Organic Synthesis: Handout 1
Prof Tim Donohoe: Strategies and Taccs in Organic Synthesis: Handout 1 Organic Synthesis III 8 x 1hr Lectures: Michaelmas Term Weeks 5-8 2016 Mon at 10am; Wed at 9am Dyson Perrins lecture theatre Copies of this handout will be available at hEp://donohoe.chem.ox.ac.uk/page16/index.html 1/33 Prof Tim Donohoe: Strategies and Taccs in Organic Synthesis: Handout 1 Organic Synthesis III Synopsis 1) Introduc5on to synthesis: (i) Why do we want to synthesise molecules- what sort of molecules do we need to make? (ii) What aspects of selecvity do we need to accomplish a good synthesis (chemo-, regio- and stereoselecvity)? (iii) Protecng group chemistry is central to any syntheAc effort (examples and principles) (iv) What is the perfect synthesis (performed in industry versus academia)? 2) The chiral pool: where does absolute stereochemistry come from? 3) Retrosynthesis- learning to think backwards (revision from first and second year). Importance of making C-C bonds and controlling oxidaAon state. Umpolung 4) Some problems to think about 5) Examples of retrosynthesis/synthesis in ac5on. 6) Ten handy hints for retrosynthesis 7) Soluons to the problems Recommended books: General: Organic Chemistry (Warren et al) Organic Synthesis: The DisconnecRon Approach (S. Warren) Classics in Total Synthesis Volumes I and II (K. C. Nicolaou) The Logic of Chemical Synthesis (E. J. Corey) 2/33 View Article Online / Journal Homepage / Table of Contents for this issue 619461 Strychniqae and BYucine. Pavt XLII. 903 Prof Tim Donohoe: Strategies and Taccs in Organic Synthesis: Handout 1 (i) Why do we want to synthesise complex molecules? Isolated from the Pacific Yew in 1962 Prescribed for prostate, breast and ovarian cancer Unique mode of acRon 1x 100 year old tree = 300 mg Taxol Isolated in 1818- poisonous Stuctural elucidaon took R. -

Artificial Intelligence for Computer-Aided Synthesis
ORIGINAL RESEARCH published: 04 August 2020 doi: 10.3389/fceng.2020.00005 Artificial Intelligence for Computer-Aided Synthesis In Flow: Analysis and Selection of Reaction Components Pieter P. Plehiers 1,2, Connor W. Coley 1, Hanyu Gao 1, Florence H. Vermeire 1, Maarten R. Dobbelaere 2, Christian V. Stevens 3, Kevin M. Van Geem 2* and William H. Green 1* 1 Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA, United States, 2 Laboratory for Chemical Technology, Department of Materials, Textiles and Chemical Engineering, Ghent University, Ghent, Belgium, 3 SynBioC Research Group, Department of Green Chemistry and Technology, Faculty of Bioscience Engineering, Ghent Edited by: University, Ghent, Belgium René Schenkendorf, Technische Universitat Braunschweig, Germany Computer-aided synthesis has received much attention in recent years. It is a challenging Reviewed by: topic in itself, due to the high dimensionality of chemical and reaction space. It becomes Richard Anthony Bourne, even more challenging when the aim is to suggest syntheses that can be performed in University of Leeds, United Kingdom Alexei Lapkin, continuous flow. Though continuous flow offers many potential benefits, not all reactions University of Cambridge, are suited to be operated continuously. In this work, three machine learning models United Kingdom have been developed to provide an assessment of whether a given reaction may benefit *Correspondence: from continuous operation, what the likelihood of success in continuous flow is for a Kevin M. Van Geem [email protected] certain set of reaction components (i.e., reactants, reagents, solvents, catalysts, and William H. Green products) and, if the likelihood of success is low, which alternative reaction components [email protected] can be considered. -
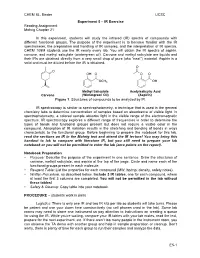
Experiment 5 – IR Exercise Reading Assignment Mohrig Chapter 21
CHEM 8L, Binder UCSC Experiment 5 – IR Exercise Reading Assignment Mohrig Chapter 21 In this experiment, students will study the infrared (IR) spectra of compounds with different functional groups. The purpose of the experiment is to become familiar with the IR spectrometer, the preparation and handling of IR samples, and the interpretation of IR spectra. CHEM 108M students use the IR nearly every lab. You will obtain the IR spectra of aspirin, carvone, and methyl salicylate (wintergreen oil). Carvone and methyl salicylate are liquids and their IRs are obtained directly from a very small drop of pure (aka “neat”) material. Aspirin is a solid and must be diluted before the IR is obtained. O O OH O O O OCH3 OH Methyl Salicylate Acetylsalicylic Acid Carvone (Wintergreen Oil) (Aspirin) Figure 1. Structures of compounds to be analyzed by IR. IR spectroscopy is similar to spectrophotometry, a technique that is used in the general chemistry labs to determine concentration of samples based on absorbance of visible light. In spectrophotometry, a colored sample absorbs light in the visible range of the electromagnetic spectrum. IR spectroscopy explores a different range of frequencies in order to determine the types of bonds and functional groups present but does not require a visible color in the compound. Absorption of IR radiation results in the stretching and bending of bonds in ways characteristic to the functional group. Before beginning to prepare the notebook for this lab, read the sections on IR in the Mohrig text and attend the IR lecture! You may bring this handout to lab to compare with literature IR, but you still need to prepare your lab notebook or you will not be permitted to enter the lab (zero points on the report). -

Diversity-Oriented Combinatorial Biosynthesis of Benzenediol Lactone Scaffolds by Subunit Shuffling of Fungal Polyketide Synthases
Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthases Yuquan Xua,b,1, Tong Zhouc,1, Shuwei Zhangc, Patricia Espinosa-Artilesb, Luoyi Wangc, Wei Zhanga, Min Lina, A. A. Leslie Gunatilakab,d, Jixun Zhanc,2, and István Molnárb,d,2 aBiotechnology Research Institute, The Chinese Academy of Agricultural Sciences, Beijing 100081, P. R. China; bNatural Products Center, School of Natural Resources and the Environment, University of Arizona, Tucson, AZ 85706; cDepartment of Biological Engineering, Utah State University, Logan, UT 84322; and dBio5 Institute, University of Arizona, Tucson, AZ 85721 Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved June 23, 2014 (received for review April 16, 2014) Combinatorial biosynthesis aspires to exploit the promiscuity of biosynthesis inhibitory activities in animals. 10,11-dehydrocurvularin microbial anabolic pathways to engineer the synthesis of new (7;Fig.1)isaDALwitha12-memberedring(DAL12)that chemical entities. Fungal benzenediol lactone (BDL) polyketides modulates the heat shock response and the immune system (8, 9). are important pharmacophores with wide-ranging bioactivities, BDL scaffolds are biosynthesized by pairs of collaborating, including heat shock response and immune system modulatory sequentially acting iterative polyketide synthases (iPKSs) (3) – effects. Their biosynthesis on a pair of sequentially acting iterative forming quasi-modular BDL synthases (BDLSs) (Fig. 1) (11 14). polyketide synthases (iPKSs) offers a test case for the modulariza- Each of the BDLS subunits catalyze recursive, decarboxylative tion of secondary metabolic pathways into “build–couple–pair” Claisen condensations of malonyl-CoA using a single core set of ketoacyl synthase (KS), acyl transferase (AT), and acyl carrier combinatorial synthetic schemes. -

Experiment 19 — Aldol Condensation
Chem 22 Spring 2010 Experiment 19 — Aldol Condensation _____________________________________________________________________________ Pre-lab preparation. (1) Write the mechanism of the base-catalyzed aldol condensation of acetone and a generalized aromatic aldehyde, Ar–CH=O to give the α,β-unsaturated product (i.e. the reaction at the top of the next page, but with a 1:1 ratio of ketone and aldehyde). Remember that dehydration in this case occurs under basic conditions, so it can't start with protonation of the hydroxyl group. Nor can it go via an E2 pathway. (2) Draw the structures of all the possible aldehyde and ketone reactants (not the 25 possible condensation products!). (3) The new CC double bonds of the condensation products are E rather than Z, as shown in the acetone example. Why? (4) What's the purpose of rinsing the crude product with dilute acetic acid, followed by ethanol? (5) What is the procedure for carrying out a single-solvent recrystallization? Write this out in detail. The aldol condensation has historically been one of the favorite tools in the synthetic organic chemist's repertoire because of its versatility in forming new CC bonds. Since its discovery in the 1870s the aldol condensation has been use extensively in the synthesis of natural products and other complex molecules. In a typical base-catalyzed aldol condensation an enolate ion attacks the carbonyl group of an aldehyde or ketone. This carbonyl addition produces a β-hydroxy carbonyl compound. In many cases the initially formed condensation product undergoes an "E1cB" dehydration to produce an α,β-unsaturated carbonyl compound as the final product. -

Retrosynthetic Design of Metabolic Pathways to Chemicals Not Found in Nature
Retrosynthetic design of metabolic pathways to chemicals not found in nature The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Lin, Geng-Min et al. "Retrosynthetic design of metabolic pathways to chemicals not found in nature." Biology 14 (April 2019): 82-107 © 2019 The Authors As Published http://dx.doi.org/10.1016/J.COISB.2019.04.004 Publisher Elsevier BV Version Final published version Citable link https://hdl.handle.net/1721.1/125854 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ Available online at www.sciencedirect.com Current Opinion in ScienceDirect Systems Biology Retrosynthetic design of metabolic pathways to chemicals not found in nature Geng-Min Lin1, Robert Warden-Rothman1 and Christopher A. Voigt Abstract simpler chemical building blocks derived from pe- Biology produces a universe of chemicals whose precision and troleum or other sources [1]. Chemicals that are large complexity is the envy of chemists. Over the last 30 years, the and complex with many functional groups and ster- expansive field of metabolic engineering has many successes eocenters have required Herculean efforts to build; in optimizing the overproduction of metabolites of industrial in- for example, halichondrin B has 32 stereocenters (4 terest, including moving natural product pathways to production billion isomers) and requires a sprawling total syn- hosts (e.g., plants to yeast). However, there are stunningly few thesis whose longest linear path is 47 reactions [2]. examples where enzymes are artificially combined to make a Solutions have been found to incredibly challenging chemical that is not found somewhere in nature. -

Organic Synthesis: New Vistas in the Brazilian Landscape
Anais da Academia Brasileira de Ciências (2018) 90(1 Suppl. 1): 895-941 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201820170564 www.scielo.br/aabc | www.fb.com/aabcjournal Organic Synthesis: New Vistas in the Brazilian Landscape RONALDO A. PILLI and FRANCISCO F. DE ASSIS Instituto de Química, UNICAMP, Rua José de Castro, s/n, 13083-970 Campinas, SP, Brazil Manuscript received on September 11, 2017; accepted for publication on December 29, 2017 ABSTRACT In this overview, we present our analysis of the future of organic synthesis in Brazil, a highly innovative and strategic area of research which underpins our social and economical progress. Several different topics (automation, catalysis, green chemistry, scalability, methodological studies and total syntheses) were considered to hold promise for the future advance of chemical sciences in Brazil. In order to put it in perspective, contributions from Brazilian laboratories were selected by the citations received and importance for the field and were benchmarked against some of the most important results disclosed by authors worldwide. The picture that emerged reveals a thriving area of research, with new generations of well-trained and productive chemists engaged particularly in the areas of green chemistry and catalysis. In order to fulfill the promise of delivering more efficient and sustainable processes, an integration of the academic and industrial research agendas is to be expected. On the other hand, academic research in automation of chemical processes, a well established topic of investigation in industrial settings, has just recently began in Brazil and more academic laboratories are lining up to contribute. -

Aldol Condensation- Aldehyde (Or Ketone) Enolate Condenses with Aldehyde (Or Ketone)
Chem 232 Summary of Alpha Substitutions page 1 Aldol Condensation- aldehyde (or ketone) enolate condenses with aldehyde (or ketone): O CH O O H 3 H CH 3 CH3 C C C C CH C C CH2 CH2 H -H O H H O OH 2 H nucleophile electrophile -hydroxy aldehyde -unsaturated aldehyde The nucleophile can be a ketone enolate or aldehyde enolate and the electrophile (shaded) can be an aldehyde or ketone. Crossed Aldol- Condensation between two different carbonyls. The component without hydrogens is the electrophile: O O O OH O C CH C C CH CH3 C H CH -H2O CH 2 CH3 H 3 ketone enolate no -hydrogens -unsaturated ketone Aldol Cyclizations- A dicarbonyl produces an enolate and carbonyl in the same molecule: enolate from a O OH 1,5-diketone CH OH 3 CH3 O O -H2O O CH2 CH3 CH3 CH2 O Claisen Condensation- Similar to Aldol condensation except the nucleophile is an ester enolate; O O O O O O + EtO C CH C OEt EtO C CH2 C EtO C CH2 CH3 C OEt 2 ketoester CH3 CH3 Dieckmann Cyclization- Internal Claisen condensation similar to Aldol cyclization. A 1,6 diester gives a 5-membered ring and a 1,7 diester gives a 6-membered ring: O OEt O OEt cyclic ketoester C C OEt O O Crossed Claisen- Similar to crossed Aldol- Electrophile has nohydrogens: O O O O C C EtO EtO C CH2 EtO C CH2 ketoester Variations on Crossed Claisen- ketone enolate and ester condensation. Esters, carbonates, formates and oxalates are common electrophiles: O O O O O O O O H C OEt H EtO C OEt OEt ethyl formate -ketoaldehyde diethyl carbonate -ketoester O O O O O OEt diketoester EtO C C OEt O diethyl oxalate -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3. -

Pacs by Chemical Name (Mg/M3) (Pdf)
Table 4: Protective Action Criteria (PAC) Rev 25 based on applicable 60-minute AEGLs, ERPGs, or TEELs. Values are presented in mg/m3. August 2009 Table 4 is an alphabetical listing of the chemicals in the PAC data set. It provides Chemical Abstract Service Registry Numbers (CASRNs)1, PAC values, and technical information on the source of the PAC values. Table 4 presents all values for TEEL-0, PAC-1, PAC-2, and PAC-3 in mg/m3. The conversion of ppm to mg/m3 is calculated assuming 25 ºC and 760 mm Hg. The columns presented in Table 4 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this alphabetical listing. Chemical Name The chemical name given to the PAC Development Team. CASRN The Chemical Abstract Service Registry Number for this chemical. TEEL-0 This is the threshold concentration below which most people will experience no adverse health effects. This PAC is always based on TEEL-0 because AEGL-0 or ERPG-0 values do not exist. PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value. PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value. PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value. Source of PACs: Technical comments provided by the PAC development team that TEEL-0, PAC-1, indicate the source of the data used to derive PAC values. Future efforts PAC-2, PAC-3 are directed at reviewing, revising, and enhancing this information. -

Highly Enantioselective Synthesis of Γ-, Δ-, and E-Chiral 1-Alkanols Via Zr-Catalyzed Asymmetric Carboalumination of Alkenes (ZACA)–Cu- Or Pd-Catalyzed Cross-Coupling
Highly enantioselective synthesis of γ-, δ-, and e-chiral 1-alkanols via Zr-catalyzed asymmetric carboalumination of alkenes (ZACA)–Cu- or Pd-catalyzed cross-coupling Shiqing Xu, Akimichi Oda, Hirofumi Kamada, and Ei-ichi Negishi1 Department of Chemistry, Purdue University, West Lafayette, IN 47907 Edited by Chi-Huey Wong, Academia Sinica, Taipei, Taiwan, and approved May 2, 2014 (received for review January 21, 2014) Despite recent advances of asymmetric synthesis, the preparation shown in Schemes 1 and 2 illustrate the versatility of ZACA of enantiomerically pure (≥99% ee) compounds remains a chal- represented by the organoaluminum functionality of the ini- lenge in modern organic chemistry. We report here a strategy tially formed ZACA products. Introduction of the OH group for a highly enantioselective (≥99% ee) and catalytic synthesis by oxidation of initially formed alkylalane intermediates in of various γ- and more-remotely chiral alcohols from terminal Scheme 2 is based on two considerations: (i) the proximity of the alkenes via Zr-catalyzed asymmetric carboalumination of alkenes OH group to a stereogenic carbon center is highly desirable for (ZACA reaction)–Cu- or Pd-catalyzed cross-coupling. ZACA–in situ lipase-catalyzed acetylation to provide ultrapure (≥99% ee) di- oxidation of tert-butyldimethylsilyl (TBS)-protected ω-alkene-1-ols functional intermediates, and (ii) the versatile OH group can R S α ω produced both ( )- and ( )- , -dioxyfunctional intermediates (3) be further transformed to a wide range of carbon groups by – ee ≥ ee in 80 88% , which were readily purified to the 99% level tosylation or iodination followed by Cu- or Pd-catalyzed cross- by lipase-catalyzed acetylation through exploitation of their high α ω coupling.