Iron Nano-Complexes and Iron Chelate Improve Biological Activities of Sweet Basil (Ocimum Basilicum L.) T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Developments in the Use of Spices Dr David Baines Baines Food Consultancy Ltd
EUROPEAN SPICE ASSOCIATION GENERAL ASSEMBLY 2013 Technical Developments in the Use of Spices Dr David Baines Baines Food Consultancy Ltd Co-editor: Flavour Horizons TECHNICAL DEVELOPMENTS IN THE USE OF SPICES TOPICS: Recent health claims submitted to the EU for the use of spices Compounds in selected spices that have beneficial effects on health The use of spices to inhibit of carcinogen formation in cooked meats The growing use of spices in animal feeds Salt reduction using spices Interesting culinary herbs from Vietnam Recent Health Claims Submitted to the EU EU REGULATION OF HEALTH CLAIMS • The Nutrition and Health Claims Regulation, 1924/2006/EC is designed to ensure a high level of protection for consumers and legal clarity and fair competition for food business operators. • Claims must not mislead consumers; they must be, accurate, truthful, understandable and substantiated by science. • Implementation of this Regulation requires the adoption of a list of permitted health claims, based on an assessment by the European Food Safety Authority (EFSA) of the science substantiating the claimed effect and compliance with the other general and specific requirements of the Regulation. • This list of permitted health claims was adopted in May 2012 by the Commission and became binding on 14th December 2012. Food companies must comply from this date or face prosecution for misleading marketing. APPROVAL OF CLAIMS EU REGULATION OF HEALTH CLAIMS CLAIMS BY COMPONENT CLAIMS BY FUNCTION CLAIMS FOR SPICES – NOT APPROVED/ON HOLD SPICE CLAIM(S) Anise / Star Anise Respiratory Health, Digestive Health, Immune Health, Lactation Caraway Digestive Health, Immune Health, Lactation Cardamon Respiratory Health, Digestive Health, Immune Health, Kidney Health, Nervous System Health, Cardiovascular Health, Capsicum Thermogenesis, Increasing Energy Expenditure, Enhancing Loss of Calories, Body Weight Loss, Stomach Health, Reduction of Oxidative Stress, promotion of Hair Growth. -

World Journal of Pharmaceutical Research SJIF Impact Factor 5.990 Volume 4, Issue 7, 1269-1300
World Journal of Pharmaceutical Research SJIF Impact Factor 5.990 Volume 4, Issue 7, 1269-1300. Review Article ISSN 2277– 7105 LIMNOPHILA (SCROPHULARIACEAE): CHEMICAL AND PHARMACOLOGICAL ASPECTS Rajiv Roy1, Shyamal K. Jash2, Raj K. Singh3 and Dilip Gorai1* 1Assistant Professor, Department of Chemistry, Bolpur College, West Bengal, India. 2Department of Chemistry, Saldiha College, Saldiha, Bankura-722 173, West Bengal, India. 3Officer-in-Charge, Mangolkot Government College, Mangolkot, West Bengal, India. ABSTRACT Article Received on 28 April 2015, The present resume covers an up-to-date and detailed literature on Limnophila species (family: Scrophulariaceae) and the botanical Revised on 22 May 2015, Accepted on 14 June 2015 classification, ethno-pharmacology, chemical constituents as well as the biological activities and pharmacological applications of both *Correspondence for isolated phytochemicals and plant extracts. There are about forty plant Author species belonging to this genus. Various classes of chemical Dr. Dilip Gorai constituents like flavonoids, terpenoids, amino acids etc. have been Assistant Professor, reported from the genus. Crude plant extracts and the isolated chemical Department of Chemistry, constituents exhibited different biological activities such as Bolpur College, West Bengal, India. antimicrobial, anti-inflammatory, anti-oxidant, cytotoxic, wound healing, hypotensive activity etc. The review covers literature upto September, 2014 enlisting 131 chemical constituents and citing 88 references. KEYWORDS: Biological -
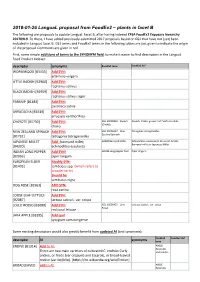
2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species
medicines Article Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species Noémi Koczka 1,*, Éva Stefanovits-Bányai 2 and Attila Ombódi 1 1 Institute of Horticulture, Szent István University, Páter K. street 1, 2100 Gödöll˝o,Hungary; [email protected] 2 Department of Applied Chemistry, Szent István University, Villányi street 29-43, 1118 Budapest, Hungary; [email protected] * Correspondence: [email protected]; Tel.: +36-28-522-000 Received: 30 June 2018; Accepted: 31 July 2018; Published: 4 August 2018 Abstract: Background: Rosehips, the fruits of Rosa species, are well known for their various health benefits like strengthening the immune system and treating digestive disorders. Antioxidant, anti-inflammatory, and cell regenerative effects are also among their health enhancing impacts. Rosehips are rich in compounds having antioxidant properties, like vitamin C, carotenoids, and phenolics. Methods: Total polyphenol content (Folin-Ciocalteu’s method), and in vitro total antioxidant capacity (ferric-reducing ability of plasma, FRAP) in rosehips of four Rosa species (R. canina, R. gallica, R. rugosa, R. spinosissima) were determined and compared. Ripe fruits were harvested at two locations. Water and ethanolic extracts of dried fruit flesh were analyzed. Results: R. spinosissima had the highest total phenolic content and antioxidant capacity, significantly higher than the other investigated Rosa species. Both parameters were reported in decreasing order for R. spinosissima > R. canina > R. rugosa > R. gallica. Ethanolic extracts of rosehips showed higher phenolic content and antioxidant activity than water extracts. Antioxidant properties were influenced by the growing site of Rosa species. Conclusions: This study indicates that R. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

Polyphenols and Rosmarinic Acid Contents, Antioxidant and Anti- Inflammatory Activities of Different Solvent Fractions from Nga- Mon (Perilla Frutescens) Leaf
Journal of Pharmacy and Nutrition Sciences, 2019, 9, 000-000 1 Polyphenols and Rosmarinic acid Contents, Antioxidant and Anti- Inflammatory Activities of Different Solvent Fractions from Nga- Mon (Perilla frutescens) Leaf Kanokkarn Phromnoi1, Maitree Suttajit1 and Chalermpong Saenjum2,3,* 1Division of Biochemistry, School of Medical Sciences, University of Phayao, Mueang, Phayao, 56000, Thailand 2Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, 50200, Thailand 3Cluster of Excellence on Biodiversity based Economics and Society (B.BES-CMU), Chiang Mai University, Chiang Mai, 50200, Thailand Abstract: Perilla is a rich source of polyphenols, which exhibits antioxidant, anti-inflammatory activities, and a variety of biological effects. The effect of differential solvents on the polyphenols, flavonoids, rosmarinic acid (RA), anti- inflammatory and antioxidant activities of perilla leaf require investigation. In this study, perilla leaf was extracted with 70% ethanol and sequentially fractionated according to the solvent’s polarity with hexane, dichloromethane, ethyl acetate, and water. Samples were subjected to the bioactive compound measurements. The antioxidant and anti- + - inflammation nature of perilla was analyzed based on the scavenging effects on DPPH•, ABTS• , O2• and nitric oxide (NO), as well as FRAP assay, and determination of the inhibition effects on NO, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) production in the cell-based study. The results indicate that among all different solvents used for sequential fractionation, ethyl acetate (EtOAc) was most effective in the separation of anti-oxidative and anti- inflammatory compounds in the perilla leaf extract. These properties can partly be due to the presence of polyphenolics, flavonoids, and also RA. -

INDEX for 2011 HERBALPEDIA Abelmoschus Moschatus—Ambrette Seed Abies Alba—Fir, Silver Abies Balsamea—Fir, Balsam Abies
INDEX FOR 2011 HERBALPEDIA Acer palmatum—Maple, Japanese Acer pensylvanicum- Moosewood Acer rubrum—Maple, Red Abelmoschus moschatus—Ambrette seed Acer saccharinum—Maple, Silver Abies alba—Fir, Silver Acer spicatum—Maple, Mountain Abies balsamea—Fir, Balsam Acer tataricum—Maple, Tatarian Abies cephalonica—Fir, Greek Achillea ageratum—Yarrow, Sweet Abies fraseri—Fir, Fraser Achillea coarctata—Yarrow, Yellow Abies magnifica—Fir, California Red Achillea millefolium--Yarrow Abies mariana – Spruce, Black Achillea erba-rotta moschata—Yarrow, Musk Abies religiosa—Fir, Sacred Achillea moschata—Yarrow, Musk Abies sachalinensis—Fir, Japanese Achillea ptarmica - Sneezewort Abies spectabilis—Fir, Himalayan Achyranthes aspera—Devil’s Horsewhip Abronia fragrans – Sand Verbena Achyranthes bidentata-- Huai Niu Xi Abronia latifolia –Sand Verbena, Yellow Achyrocline satureoides--Macela Abrus precatorius--Jequirity Acinos alpinus – Calamint, Mountain Abutilon indicum----Mallow, Indian Acinos arvensis – Basil Thyme Abutilon trisulcatum- Mallow, Anglestem Aconitum carmichaeli—Monkshood, Azure Indian Aconitum delphinifolium—Monkshood, Acacia aneura--Mulga Larkspur Leaf Acacia arabica—Acacia Bark Aconitum falconeri—Aconite, Indian Acacia armata –Kangaroo Thorn Aconitum heterophyllum—Indian Atees Acacia catechu—Black Catechu Aconitum napellus—Aconite Acacia caven –Roman Cassie Aconitum uncinatum - Monkshood Acacia cornigera--Cockspur Aconitum vulparia - Wolfsbane Acacia dealbata--Mimosa Acorus americanus--Calamus Acacia decurrens—Acacia Bark Acorus calamus--Calamus -

Microwave-Assisted Hydrodistillation: Garlic Essential Oil Recovery
Faculty of Engineering and Science Microwave-Assisted Hydrodistillation: Garlic Essential Oil Recovery Ivy Yii Siew Aing This thesis is presented for the Degree of Master of Philosophy (Chemical Engineering) of Curtin University November 2017 Declaration To the best of my knowledge and belief this thesis contains no material previously published by any other person except where due acknowledgment has been made. This thesis contains no material, which has been accepted for the award of any other degree or diploma in any university. Signature: …………………………………………. Date: ………………………... ACKNOWLEDGEMENT This thesis has become a reality with the support and assistance from help of many individuals and departments. I would like to express my deepest appreciation to all who encouraged and supported me to accomplish this feat. First and foremost, I wish to thank my supervisor, Associate Professor Jobrun Nandong. It was due to his valuable guidance, enthusiasm, selfless help and patience that I was able to complete my research work. I am very thankful to him because his guidance has helped me to finish my experimental and modelling work on time. I also wish to express my deepest gratitude to my supervisor, Dr. Stephanie Chan Yan San, who inspired and encouraged me with her words and blessings. She provided critical and useful advice especially in improving the methodologies for my experiments and draw inferences from my research data. Her willingness to continue supervising my work motivated me to complete this thesis. Special thanks to Associate Professor Chua Han Bing and Professor Michael K Danquah who also provided me with valuable guidance and suggestions over the years. -

Dietary Intake of Spices and Herbs in Habitual Northeast Thai Diets
Mal J Nutr 16(1): 137 - 148, 2010 Dietary Intake of Spices and Herbs in Habitual Northeast Thai Diets Tantipopipat S, Boonpraderm A, Charoenkiatkul S, Wasantwisut E & Winichagoon P Institute of Nutrition, Mahidol University (INMU), Salaya, Nakhon Pathom, 73170 Thailand ABSTRACT Spices and herbs are rich in polyphenols and widely used in habitual diets in the tropical regions. To elucidate their effects on human health, intake of the portion of spices and herbs from habitual diets should be determined. Consumption patterns were determined from 24-hour records or recalls of 181 men and 370 women in Khonkaen and Ubon Ratchathani provinces, representing upper and lower northeast Thailand. There was a slight variation in dishes, but twelve spices/herbs were commonly used in the two areas. The amounts of spices/ herbs in the four most common dishes (Somtum, Jaew, Pon and Kang-Nor-Mai) were estimated by weighing ingredients before and after cooking. The average amount of spices/herbs consumed was 4.9, 26.1, 14 and 11 g/meal, contributing 36.6, 43.1, 20.6 and 29.8 mg polyphenols/meal for Somtum, Jaew, Pon and Kang- Nor-Mai, respectively. Chili was common in all recipes, with an average amount of 8.3-27.5 mg polyphenols/meal. In conclusion, habitual northeast Thai diets contain several spices/herbs and a substantial amount of polyphenols was commonly consumed. Keywords: Herbs, Northeast diets, polyphenols, portion size, spices, Thailand INTRODUCTION and spice, depending on the parts used. For example, coriander leaf is an herb, while its Spices and herbs have long been used as dried seed is a spice. -

2013 Annual Meeting Abstracts Analytical
2013 Annual Meeting Abstracts Analytical MONDAY AFTERNOON ANA 1/LOQ 1.1: Marine Oils - Analytical and Stability Chair(s): J. Reuther, Eurofins Central Analytical Labs, USA; V. Barthet, Canadian Grain Commission, Canada Quantitation of Fatty Acids in Marine Oils by Comprehensive gc-online Hydrogenation x Gc. P. Delmonte(1), A. Fardin Kia(2), J. Rader(3) (1)US Food and Drug Administration, United States of America (2)US Food and Drug Administration, United States of America (3)US Food and Drug Administration, United States of America In this study, the separation of fatty acid methyl esters (FAME) prepared from marine oils provided by an SLB-IL111 capillary column is enhanced by adding a second dimension of separation in a comprehensive GCxGC design. After elution from the first column, the FAME are reduced to their fully saturated form by passing through a capillary tube coated with palladium in the presence of hydrogen carrier gas. The products of reduction are then separated by the secondary high polarity capillary column. The two dimensional separations obtained using this technique can be easily interpreted based on the principle that all the saturated FAME lie on a straight line bisecting the separation plane, while the FAME with the same carbon skeleton but differing in the number, geometric configuration or position of double bonds lie on lines parallel to the D1 time axis. This methodology provides the quantitation of the FAME with different chain lengths that are not separated by mono-dimensional chromatography and it also provides valuable structural information without use of a mass spectrometer. The ease of interpretation of the two dimensional chromatograms and the higher separation capability make this technique far superior to the most refined mono-dimensional separations of FAME. -

Limnophila (Scrophulariaceae): Chemical and Pharmaceutical Aspects - an Update
Send Orders for Reprints to [email protected] The Open Natural Products Journal, 2014, 7, 1-14 1 Open Access Limnophila (Scrophulariaceae): Chemical and Pharmaceutical Aspects - An Update Goutam Brahmachari* Laboratory of Natural Products & Organic Synthesis, Department of Chemistry, Visva-Bharati (a Central University), Santiniketan-731 235, West Bengal, India Abstract: The present resumé covers an up-to-date literature on Limnophila species. The botanical classification, ethno- pharmacology, and chemical constituents of Limnophila plants, as well as the biological activities and pharmacological applications of both distinct phytochemicals and medicinally active plant materials (formulations, extracts, etc.) are dis- cussed in detail. Keywords: Biological activities, Botany, Chemical constituents, Ethno-pharmacology, Limnophila species, Pharmacological applications, Taxonomical classification. INTRODUCTION feet long. The emergent stems are usually 2-15 cm above the surface of the water. Single white, pink, purple or blue to Limnophila (family: Scrophulariaceae) [1-5] is originated lavender flowers, sometimes with conspicuous spots, occa- from a Latin word that means pond-loving indicating its ex- sionally occur on the emerged portion of the stem. The flow- istence in aquatic environments. It is commonly known as ers are stalkless and borne in the leaf axis, and are axillary ‘Ambulia’ (Asian marshweed). It is a perennial herb from and solitary or in axillary or terminal spikes or racemes, Southeast Asia, tropical to subtropical Africa, Australia, and sessile or pedicellate. The lower portion (sepals) has five, Pacific Islands; also finds adventive distribution in North green, hairy lobes, each 4-5 mm long. The upper portion is America. Limnophila plants are widely distributed through- purple and composed of five fused petals forming a tube out India, and occupy a significant position in traditional with two lips — adaxial lip (dorsal) is 2-lobed, while abaxial systems of medicine. -

In Vitro Comparative Assessment of the Inhibitory Effects of Single and Combined Spices Against Glucose-Synthesizing Enzymes
Adeyeoluwa et al Tropical Journal of Pharmaceutical Research June 2020; 19 (6): 1209-1214 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v19i6.14 Original Research Article In vitro comparative assessment of the inhibitory effects of single and combined spices against glucose-synthesizing enzymes Temitayo Esther Adeyeoluwa, Fatai Oladunni Balogun, Anofi Omotayo Tom Ashafa* Phytomedicine and Phytopharmacology Research Group, Department of Plant Sciences, University of the Free State, QwaQwa Campus, Private Bag x13, Phuthaditjhaba 9866, QwaQwa, Free State, South Africa *For correspondence: Email: [email protected] Sent for review: 15 November 2019 Revised accepted: 23 May 2020 Abstract Purpose: To assess the individual and synergistic inhibitory activities of five (5) tropical spices, viz, Zingiber officinale Rosc., Xylopia aethiopica (Dun.) A. Rich, Piper guineense Schum & Thonn, Myristica fragrans Houtt. and Allium sativum L. against glucose hydrolysing enzymes. Methods: In vitro assessment of the alpha-amylase and alpha-glucosidase inhibitory activities of cold water, hot water, ethanol and aqueous-ethanol extracts of each of the five spices and their combination was investigated using enzymatic protocols. Acarbose was used as the standard. Results: Only four extracts showed strong inhibition against alpha-glucosidase and weak inhibition against alpha-amylase. Going by their half-maximal inhibitory concentrations (IC50), the combined spices exhibited the best concurrent inhibitory activity against both hydrolysing enzymes (665.83 ± 27.50 µg/mL for alpha-glucosidase) and (1166.86 ± 14.70 µg/mL for alpha-amylase).