Bismuth Sulfite Agar, Product Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Culture Media Edition for Industrial Microbiology LABORATORIOS CONDA S.A
2nd Edition for Industrial Microbiology Culture Media LABORATORIOS CONDA S.A. Edited by: Laboratorios Conda S.A. © 2013. Conda S.A. All rights reserved. Printed in Spain C/ La Forja, 9 28850 - Torrejón de Ardoz, Madrid - SPAIN Tel. +34 91 761 02 00 Fax +34 91 656 82 28 C/ Berlín, 63 08029 Barcelona - SPAIN Tel. +34 93 363 72 64 / 65 Fax. +34 93 363 72 61 [email protected] [email protected] www.condalab.com Index 6 Meat & Fish Industry 20 Beer Industry 10 Water & Beverages 21 Waste Water 11 Dairy Products 23 Cosmetic Industry 15 Bakery 24 Pharmaceutical Industry 17 Processed Foods 25 Microbiology Dehydrated Culture Media Guide 19 Wines iv Media for Industrial Microbiology CULTURE MEDIA FOR INDUSTRIAL MICROBIOLOGY | 2ND EDITION Media for Industrial Microbiology 5 Culture Media for Industrial Microbiology Laboratorios CONDA, one of the world USP and AOAC standards. Strict quality leaders in the design and manufacturing of control procedures are adopted prior to, high quality culture media, currently offers during and after the manufacturing process more than 400 different products, among to ensure quality products and batch-to-batch which you will find chromogenic media, ISO- consistency. We also exert tight control over formulated media and custom-made media selection and treatment of all raw materials for many different industrial applications. and components (peptones, carbohydrates, minerals, chemicals, agar and other additives) From hygiene control, through food used in the manufacturing process. Physical- and beverage poisoning prevention, to chemical characteristics are tested, and microbiologial examination of cosmetic and media also undergo additional microbiological pharmaceutical products, CONDA supplies a tests that guarantee growth, differentiation, wide variety of different media for each field biochemical performance, recovery of small so that customers can find the most suitable inocula, selectivity, etc. -

PDF, Effect of Differences in Salt Concentration on the Quality Of
IOP Conference Series: Earth and Environmental Science PAPER • OPEN ACCESS Effect of Differences in Salt Concentration on the Quality of Rebon Shrimp Paste (Acetes Sp) in Tegal District To cite this article: S Mulyani et al 2021 IOP Conf. Ser.: Earth Environ. Sci. 755 012051 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.33.19 on 26/09/2021 at 20:52 ACHOST 2020 IOP Publishing IOP Conf. Series: Earth and Environmental Science 755 (2021) 012051 doi:10.1088/1755-1315/755/1/012051 Effect of Differences in Salt Concentration on the Quality of Rebon Shrimp Paste (Acetes Sp) in Tegal District S Mulyani 1*, P M Vestiyati 1, Kusnandar 1, H K Alamsyah 1, and S W Simanjuntak 1 1Faculty of Fisheries and Marine Science, Pancasakti University of Tegal, Indonesia *[email protected] Abstract. Rebon Shrimp Paste (RSP) in Indonesia uses different percentages of salt addition, ranging from 2 to 20% or not at all. This study aims to determine the influence of different salt concentration (5%, 10%, 15% and without salt) on the quality of RSP organoleptic, microbiological and chemical. This research was conducted in Munjung Agung, Tegal and Cirebon Fisheries Product Quality Testing and Application Laboratory. The results showed that the addition of different salt concentration (5%, 10%,15% and without salt) affected the quality of organoleptics, microbiology, and chemistry. Organoleptic quality with salt concentration of 5% and 10% favored panelists with an average value of 6.8 (not yet meeting Indonesian National Standards). The highest water content value is found in RSP that are not added salt (40,19%-43,22%) and lowest at 15% salt concentration (31,12%-34,82%) in accordance with the SNI. -

Food Microbiology
Food Microbiology Food Water Dairy Beverage Online Ordering Available Food, Water, Dairy, & Beverage Microbiology Table of Contents 1 Environmental Monitoring Contact Plates 3 Petri Plates 3 Culture Media for Air Sampling 4 Environmental Sampling Boot Swabs 6 Environmental Testing Swabs 8 Surface Sanitizers 8 Hand Sanitation 9 Sample Preparation - Dilution Vials 10 Compact Dry™ 12 HardyCHROM™ Chromogenic Culture Media 15 Prepared Media 24 Agar Plates for Membrane Filtration 26 CRITERION™ Dehydrated Culture Media 28 Pathogen Detection Environmental With Monitoring Contact Plates Baird Parker Agar Friction Lid For the selective isolation and enumeration of coagulase-positive staphylococci (Staphylococcus aureus) on environmental surfaces. HardyCHROM™ ECC 15x60mm contact plate, A chromogenic medium for the detection, 10/pk ................................................................................ 89407-364 differentiation, and enumeration of Escherichia coli and other coliforms from environmental surfaces (E. coli D/E Neutralizing Agar turns blue, coliforms turn red). For the enumeration of environmental organisms. 15x60mm plate contact plate, The media is able to neutralize most antiseptics 10/pk ................................................................................ 89407-354 and disinfectants that may inhibit the growth of environmental organisms. Malt Extract 15x60mm contact plate, Malt Extract is recommended for the cultivation and 10/pk ................................................................................89407-482 -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Florula of Larval and Imaginal Phases of the Volfartova Fly (Wohlfarthia magnifica) In the Conditions of the Steppe Zone of The Pavlodar Region. A A Bitkeyeva1* and L T Bulekbayeva2. 1Senior teacher, Master of Ecology, Pavlodar State University named after S. Toraygyrov, The Republic of Kazakhstan. 2Associate professor, Candidate of Biological Sciences, Pavlodar State Pedagogical Institute, Republic of Kazakhstan. ABSTRACT Groups of bacteria were found during research in a steppe zone of the Pavlodar region, belonging to 3 families: Baccilaceae, Micrococcaceae, Enterobacteriacea. 13 species of pathogenic and opportunistic bacteria are obtained and identified, which cause diseases. Reception of agents from flies of Wohlfartia magnifica family in region farms forces to pay attention to quite real possibility and contagion of various infections. It creates the menacing epidemiological and epizootiology situation on the adjacent to farms of populated places, as flies with excrements can infect forages and migrate on considerable distances. Keywords: bacteria, diseases, infections, larvaes, microorganisms, flies, sheep, pathogenic microorganisms, carriers. *Corresponding author July– August 2015 RJPBCS 6(4) Page No. 2069 ISSN: 0975-8585 INTRODUCTION Flies are known as carriers of causative agents of dangerous infectious and invasive diseases. Therefore, in the populated places and on the pastures, studying of microbal and helminthosis impurity of flies represents scientific and practical interest. Epidemiological value of flies was opened by E.N. Pavlovskiy and V.P. Derbeneva-Ukhova, they participate in distribution about 70 pathogenic microflora, and including agents of a tularemia, anthrax, diphtheria, cholera, plague, a crab hand, etc. [2; 8; 12]. -

Clinical Microbiology 12Th Edition
Volume 1 Manual of Clinical Microbiology 12th Edition Downloaded from www.asmscience.org by IP: 94.66.220.5 MCM12_FM.indd 1 On: Thu, 18 Apr 2019 08:17:55 2/12/19 6:48 PM Volume 1 Manual of Clinical Microbiology 12th Edition EDITORS-IN-CHIEF Karen C. Carroll Michael A. Pfaller Division of Medical Microbiology, Departments of Pathology and Epidemiology Department of Pathology, The Johns Hopkins (Emeritus), University of Iowa, University School of Medicine, Iowa City, and JMI Laboratories, Baltimore, Maryland North Liberty, Iowa VOLUME EDITORS Marie Louise Landry Robin Patel Laboratory Medicine and Internal Medicine, Infectious Diseases Research Laboratory, Yale University, New Haven, Connecticut Mayo Clinic, Rochester, Minnesota Alexander J. McAdam Sandra S. Richter Department of Laboratory Medicine, Boston Department of Laboratory Medicine, Children’s Hospital, Boston, Massachusetts Cleveland Clinic, Cleveland, Ohio David W. Warnock Atlanta, Georgia Washington, DC Downloaded from www.asmscience.org by IP: 94.66.220.5 MCM12_FM.indd 2 On: Thu, 18 Apr 2019 08:17:55 2/12/19 6:48 PM Volume 1 Manual of Clinical Microbiology 12th Edition EDITORS-IN-CHIEF Karen C. Carroll Michael A. Pfaller Division of Medical Microbiology, Departments of Pathology and Epidemiology Department of Pathology, The Johns Hopkins (Emeritus), University of Iowa, University School of Medicine, Iowa City, and JMI Laboratories, Baltimore, Maryland North Liberty, Iowa VOLUME EDITORS Marie Louise Landry Robin Patel Laboratory Medicine and Internal Medicine, Infectious Diseases Research Laboratory, Yale University, New Haven, Connecticut Mayo Clinic, Rochester, Minnesota Alexander J. McAdam Sandra S. Richter Department of Laboratory Medicine, Boston Department of Laboratory Medicine, Children’s Hospital, Boston, Massachusetts Cleveland Clinic, Cleveland, Ohio David W. -

BD Industry Catalog
PRODUCT CATALOG INDUSTRIAL MICROBIOLOGY BD Diagnostics Diagnostic Systems Table of Contents Table of Contents 1. Dehydrated Culture Media and Ingredients 5. Stains & Reagents 1.1 Dehydrated Culture Media and Ingredients .................................................................3 5.1 Gram Stains (Kits) ......................................................................................................75 1.1.1 Dehydrated Culture Media ......................................................................................... 3 5.2 Stains and Indicators ..................................................................................................75 5 1.1.2 Additives ...................................................................................................................31 5.3. Reagents and Enzymes ..............................................................................................75 1.2 Media and Ingredients ...............................................................................................34 1 6. Identification and Quality Control Products 1.2.1 Enrichments and Enzymes .........................................................................................34 6.1 BBL™ Crystal™ Identification Systems ..........................................................................79 1.2.2 Meat Peptones and Media ........................................................................................35 6.2 BBL™ Dryslide™ ..........................................................................................................80 -

Bismuth Sulfite Agar
Bismuth Sulfite Agar Intended Use mended for use in testing clinical specimens.15,16 In addition, Bismuth Sulfite Agar is a highly selective medium used for Bismuth Sulfite Agar is valuable when investigating outbreaks of isolating Salmonella spp., particularly Salmonella Typhi, from Salmonella spp., especially S. Typhi.17-19 food and clinical specimens. Bismuth Sulfite Agar is used for the isolation of S. Typhi and other Salmonella from food, feces, urine, sewage and other Summary and Explanation infectious materials. The typhoid organism grows luxuriantly Salmonellosis continues to be an important public health on the medium, forming characteristic black colonies, while problem worldwide, despite efforts to control the prevalence of gram-positive bacteria and members of the coliform group Salmonella in domesticated animals. Infection with nontyphi are inhibited. This inhibitory action of Bismuth Sulfite Agar Salmonella often causes mild, self-limiting illness.1 Typhoid toward gram-positive and coliform organisms permits the use fever, caused by S. Typhi, is characterized by fever, headache, of a much larger inoculum than possible with other media diarrhea and abdominal pain, and can produce fatal respi- employed for similar purposes in the past. The use of larger ratory, hepatic, splenic and/or neurological damage. These inocula greatly increases the possibility of recovering the illnesses result from consumption of raw, undercooked or pathogens, especially when they are present in relatively small improperly processed foods contaminated with Salmonella. numbers. Small numbers of organisms may be encountered in Many cases of Salmonella-related gastroenteritis are due to the early course of the disease or in the checking of carriers improper handling of poultry products. -
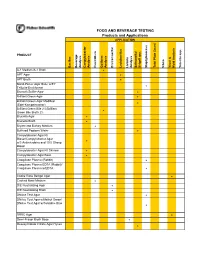
DONE Food and Beverage Testing
FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. A-1 Medium/A-1 Broth ! APT Agar ! APT Broth ! Baird-Parker Agar Base w/EY Tellurite Enrichment ! Bismuth Sulfite Agar ! Brilliant Green Agar ! Brilliant Green Agar Modified (Edel-Kampelmacher) ! Brilliant Green Bile 2%/Brilliant Green Bile Broth 2% ! Brucella Agar ! Brucella Broth ! Bryant and Burkey Medium ! Buffered Peptone Water ! Campylobacter Agar Kit Blaser/Campylobacter Agar w/5 Antimicrobics and 10% Sheep ! Blood Campylobacter Agar Kit Skirrow ! Campylobacter Agar Base ! Coagulase Plasma (Rabbit) ! Coagulase Plasma EDTA (Rabbit)/ Coagulase Plasma w/EDTA ! Cooke Rose Bengal Agar ! Cooked Meat Medium ! D/E Neutralizing Agar ! D/E Neutralizing Broth ! DNAse Test Agar ! DNAse Test Agar w/Methyl Green/ DNAse Test Agar w/Toluidine Blue ! DRBC Agar ! Demi-Fraser Broth Base ! Desoxycholate Citrate Agar Hynes ! FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. Differential Reinforced Clostridial Agar ! EC Medium/EC Broth ! EC Medium with MUG/EC Broth w/MUG ! Elliker Broth ! m Endo Agar LES ! m Endo -

Cultivation of Bacteria
CULTIVATION OF BACTERIA RAKESH SHARDA Department of Veterinary Microbiology NDVSU College of Veterinary Science & A.H., MHOW CULTIVATION OF AEROBIC BACTERIA For bacteria of veterinary importance, aerobic incubation is uniformly done at 37ºC. Depending upon the workload a laboratory may have a tabletop incubator or a walk-in incubator. For prolonged incubations, as required for the growth of Mycobacterium tuberculosis, screw-capped bottles should be used instead of Petri dishes or tubes to prevent the drying of medium. CULTIVATION OF ANAEROBIC BACTERIA Incorporation of reducing agents into the liquid medium Freshly steamed liquid media are at least temporarily anaerobic, but soon become aerobic unless a reducing agent is added.. Reducing agents include glucose 0.5-1%, ascorbic acid 0.1%, cysteine 0.1%, sodium mercaptoacetate or thioglycollate 0.1%, or the particles of meat in cooked meat broth. Examples - Thioglycollate broth, Robertson's cooked meat medium Addition of 0.05-0.1% agar can further increase the effectiveness of reducing agents Liquid media should be `pre-reduced' by holding in a boiling water bath for 10 min, to drive off dissolved oxygen Incorporation of reducing agents in Petri dish lids (Brewer’s method) a simple and safe method of obtaining anaerobiasis when using solid medium sodium dithionite (reducing agent), together with sodium bicarbonate and sodium carbonate (to supply CO2), is added in the lid of a Petri dish sodium dithionite removes the oxygen to give anaerobic conditions Displacement of oxygen by inert gases Anaerobic jar using hydrogen Most of the air from the anaerobic jar is removed and replaced with hydrogen or preferably hydrogen mixed with nitrogen in the presence of a catalyst The hydrogen reacts with the remaining oxygen to form water A common catalyst used is palladium, which should be completely dried Anaerobiasis is checked by a chemical indicator and also a bacterial indicator Metal anaerobic jar (McIntosh and Filde’s jar) 1. -

Download the Acumedia Cross Reference Guide
ACUMEDIA BD/DIFCO BD/BBL OXOID MERCK * Similar formula, but not exact + Supplement required. 2xYT Medium (7281) 244020 4.85008 A-1 Medium (7601) 218231 1.00415 Acutone TSB (7729) VG0101 1.00525 Agar, Bacteriological (7178) 214530 299340 Agar, Select (7558) 214010 212304 LP0011 1.01614 Agar, Technical (7619) 281230 LP0013 1.11925 APT Agar (7302) 265430 1.10453 Azide Dextrose Broth (7315) 238710 CM0868 1.01590 Bacillus Cereus Agar Base (7442) CM0617 + Baird Parker Agar (7112) 276840+ CM0275 + 1.05406 + BCYE Agar Base (7728) 218301 212327+ CM0655 + Beef Extract Powder (7228) 211520 212303 LP0029 1.03979 Beta-SSA Agar (7336) BIGGY Agar (7191) 211027 CM0589 1.10456 Bile Esculin Agar (7249) 299068 CM0888 Bile Esculin Azide Agar (7133) 212205* 1.00720 Bile Salts Mixture #3 (7230) 213020 LP0056 Bismuth Sulfite Agar (7113) 273300 CM0201 1.05418 Blood Agar Base No. 2 (7266) CM0271 1.10328 Blood Agar Base, Improved (7268) 211037 CM0854 1.10886 Brain Heart Infusion Agar (7115) 241830 211065 CM0225 1.13825 Brain Heart Infusion Broth (7116) 237500 211059 CM0225 1.04930 Brain Heart Infusion Solids (7262) Brilliant Green Agar (7117) 228530 CM0263 1.07232 Brill Green Agar w/ Sulfadiazine (7310) Brill Green Agar w/ Sulfapyridine (7299) 271710 1.11274 Brilliant Green Bile Broth 2% (7119) 274000 CM0031 1.05454 Brucella Agar (7120) 271000 211086 CM0169 1.10490 Brucella Broth (7121) 211088 Buff Listeria Enrich Broth (7579) 220530* Buff Listeria Enrich Broth Base (7675) 290720 CM0897 1.09628 Buffered Peptone Water (7418) 218105 212367 CM0509 1.07228 Buff Sod Chloride-Peptone Sol, pH 7.0 (7732) N/A N/A CM0982 1.10582 Campy Bld Free Select Med (7527) CM0739 + 1.00070+ Campy Cefex Agar (7718) Campy Selective Agar Base (7443) CM689 + 1.02248 + Campy Enrichment Broth (7526) CM0983 + 1.00068 + THE LAB DEPOT | LABDEPOTINC.COM | 800.733.2522 PAGE 1 ACUMEDIA BD/DIFCO BD/BBL OXOID MERCK * Similar formula, but not exact + Supplement required. -

CDC Anaerobe 5% Sheep Blood Agar with Phenylethyl Alcohol (PEA) CDC Anaerobe Laked Sheep Blood Agar with Kanamycin and Vancomycin (KV)
Difco & BBL Manual Manual of Microbiological Culture Media Second Edition Editors Mary Jo Zimbro, B.S., MT (ASCP) David A. Power, Ph.D. Sharon M. Miller, B.S., MT (ASCP) George E. Wilson, MBA, B.S., MT (ASCP) Julie A. Johnson, B.A. BD Diagnostics – Diagnostic Systems 7 Loveton Circle Sparks, MD 21152 Difco Manual Preface.ind 1 3/16/09 3:02:34 PM Table of Contents Contents Preface ...............................................................................................................................................................v About This Manual ...........................................................................................................................................vii History of BD Diagnostics .................................................................................................................................ix Section I: Monographs .......................................................................................................................................1 History of Microbiology and Culture Media ...................................................................................................3 Microorganism Growth Requirements .............................................................................................................4 Functional Types of Culture Media ..................................................................................................................5 Culture Media Ingredients – Agars ...................................................................................................................6 -

Evaluation of Plating Media for Recovering Salmonella from Thermally Treated Egg Albumen1
© 2009 Poultry Science Association, Inc. Evaluation of plating media for recovering 1 Salmonella from thermally treated egg albumen J. B. Gurtler2 Food Safety Intervention Technologies Research Unit, USDA, Agricultural Research Service, Eastern Regional Research Center, 600 E. Mermaid Lane, Wyndmoor, PA 19038-8551 Primary Audience: Quality Assurance Personnel, Researchers, Egg Processing Plant Managers, Regulators SUMMARY Salmonella spp. present in pasteurized egg albumen are often difficult to recover by direct plating because of thermal injury and the presence of innate iron binding and other antimicrobi- als in egg white. The literature has reported a multiplicity of selective and nonselective media used to recover heat-injured Salmonella, to measure the proportion of injured cells, or both. This study compared the proficiency of selective and nonselective plating media for support- ing colony development or for assessing bacterial injury of heat-stressed Salmonella from egg albumen. A 6-strain composite of Salmonella was added to albumen (pH 9.0), heated at 53.3°C for 3.1 min, and plated on 26 nonselective and 22 selective media. Recovery of heat-injured sal- monellae varied little (≤0.52 log cfu/mL) among the nonselective media tryptic soy agar, plate count agar, dextrose tryptone agar, or brain heart infusion agar, regardless of the manufacturer. Selective media that were optimal for recovery of salmonellae from albumen included 3 bril- liant green agars, Levine eosin methylene blue agar, and bismuth sulfite agar, which recovered more cells (P ≤ 0.05) than selenite-cystine, tetrathionate, xylose-lysine-tergitol-4 , xylose lysine deoxycholate, or Rappaport-Vassiliadis agars. The results of this study may assist in choosing selective or nonselective media to maximize the recovery of Salmonella from thermally treated albumen.