The First Genetic Linkage Map for Fraxinus Pennsylvanica and Syntenic Relationships with Four Related Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(APOCI, -C2, and -E and LDLR) and the Genes C3, PEPD, and GPI (Whole-Arm Translocation/Somatic Cell Hybrids/Genomic Clones/Gene Family/Atherosclerosis) A
Proc. Natl. Acad. Sci. USA Vol. 83, pp. 3929-3933, June 1986 Genetics Regional mapping of human chromosome 19: Organization of genes for plasma lipid transport (APOCI, -C2, and -E and LDLR) and the genes C3, PEPD, and GPI (whole-arm translocation/somatic cell hybrids/genomic clones/gene family/atherosclerosis) A. J. LUSIS*t, C. HEINZMANN*, R. S. SPARKES*, J. SCOTTt, T. J. KNOTTt, R. GELLER§, M. C. SPARKES*, AND T. MOHANDAS§ *Departments of Medicine and Microbiology, University of California School of Medicine, Center for the Health Sciences, Los Angeles, CA 90024; tMolecular Medicine, Medical Research Council Clinical Research Centre, Harrow, Middlesex HA1 3UJ, United Kingdom; and §Department of Pediatrics, Harbor Medical Center, Torrance, CA 90509 Communicated by Richard E. Dickerson, February 6, 1986 ABSTRACT We report the regional mapping of human from defects in the expression of the low density lipoprotein chromosome 19 genes for three apolipoproteins and a lipopro- (LDL) receptor and is strongly correlated with atheroscle- tein receptor as well as genes for three other markers. The rosis (15). Another relatively common dyslipoproteinemia, regional mapping was made possible by the use of a reciprocal type III hyperlipoproteinemia, is associated with a structural whole-arm translocation between the long arm of chromosome variation of apolipoprotein E (apoE) (16). Also, a variety of 19 and the short arm of chromosome 1. Examination of three rare apolipoprotein deficiencies result in gross perturbations separate somatic cell hybrids containing the long arm but not of plasma lipid transport; for example, apoCII deficiency the short arm of chromosome 19 indicated that the genes for results in high fasting levels oftriacylglycerol (17). -

Harnessing the Power of Epigenetics for Targeted Nutrition
SPONSOR FEATURE SPONSOR FEATURE NESTLÉ RESEARCH: Harnessing the power of epigenetics for targeted nutrition Authors Diet and genomes interact. Because of its contin- products that are nutritious, safe, promote and Martin Kussmann*, Jennifer Dean*, uous and lifelong impact, nutrition might be the maintain health, and prevent disease. With the Rondo P. Middleton†, Peter J. van most important environmental factor for human advent and development of many new technolo- Bladeren* & Johannes le Coutre* health. Molecular nutrition research strives gies, molecular nutrition research has opened to understand this interaction. Nutrigenetics up new doors of understanding – not only con- *Nestlé Research Center, addresses how an individual’s genetic make-up cerning which dietary components may impact 1000 Lausanne 26, Switzerland. leads to a predisposition for nutritional health; an organism’s health, but also how. These new †Nestlé Purina Research, St. Louis, MO, nutrigenomics (encompassing transcriptomics, insights have allowed a greater understanding 63164 USA. proteomics and metabonomics) asks how nutri- of the systems involved at multiple levels, from tion modulates gene expression; and epigenetics whole animal to the cellular and molecular lev- provides insights into a new level of regulation els. Although challenges in this area still remain, involving mechanisms of development, parental the main focus continues to be the improvement gene imprinting and metabolic programming that of health through diet. Modern molecular nutri- are beyond genetic control. tional research aims at health promotion, dis- Tailoring diets and nutrient compositions to the ease prevention, performance improvement and needs of consumer groups that share the same benefit-risk assessment1. health state, life stage or life style is a main goal Nutrition has conventionally been considered of current nutritional research. -

The Circular Genetic Map of Phage 813 by Ron Baker and Irwin Tessman
THE CIRCULAR GENETIC MAP OF PHAGE 813 BY RON BAKER AND IRWIN TESSMAN DEPARTMENT OF BIOLOGICAL SCIENCES, PURDUE UNIVERSITY, LAFAYETTE, INDIANA Communicated by S. E. Luria, July 24, 1967 Because of its small size1 phage S13 is suitable for a study of all its genes and their functions. With this aim, conditionally lethal mutants of the suppressible (su) and temperature-sensitive (t) type have been isolated and classified into seven complementation groups, and the general function of five of the genes implicitly defined by these complementation groups has been determined.8-10 This paper is concerned with the mapping of the seven known phage genes, with the ultimate aim of understanding both the organization of the phage genome and the mechanism by which it undergoes genetic recombination. Although the DNA molecule of S13, as shown for the closely related phage ,X174, is physically circular both in the single-stranded form of the mature virus11 and in the double-stranded replicative form,12' 13 this does not necessarily imply that the genetic map should also be circular since circular DNA is neither necessary nor sufficient for genetic circularity. On the one hand, a linear DNA molecule can give rise to a circular genetic map if the nucleotide sequences are circularly permuted;14 an example is the T2-T4 phage system.'5-17 On the other hand, a circular DNA can yield a linear map if recombination involves opening of the ring at a unique site, as seems to occur for phage X, which forms a closed DNA molecule after infections yet has a linear vegetative and prophage genetic map.19 In this report it will be shown that the genetic map of S13, as determined entirely by 3-factor crosses, is indeed circular, and, as might be expected for a circular genome, the occurrence of double recombination events appears to be the rule. -

Revision of the Genus Cleidion (Euphorbiaceae) in Malesia
BLUMEA 50: 197–219 Published on 22 April 2005 http://dx.doi.org/10.3767/000651905X623373 REVISION OF THE GENUS CLEIDION (EUPHORBIACEAE) IN MALESIA KRISTO K.M. KULJU & PETER C. VAN WELZEN Nationaal Herbarium Nederland, Universiteit Leiden branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands; e-mail: [email protected], [email protected] SUMMARY A revision of the Malesian species in the genus Cleidion is presented. Cleidion javanicum is shown to be the correct name for the widespread type species (instead of the name C. spiciflorum). A new species, C. luziae, resembling C. javanicum, is described from the Moluccas, New Guinea and the Solomon Islands. In addition, C. salomonis is synonymised with C. papuanum and C. lanceolatum is treated as a variety of C. ramosii. In total 7 Malesian Cleidion species are recognized. Cleidion megistophyllum from the Philippines cannot reliably be confirmed to belong to the genus due to lack of information and specimens and is treated as a doubtful species. Key words: Cleidion, Acalypheae, Cleidiinae, revision, taxonomy, Malesia. INTRODUCTION Cleidion is a pantropical genus belonging to the large angiosperm family Euphorbiaceae s.s. It was described by Blume (1825), who included a single species C. javanicum1. The first revision was made by Müller Argoviensis (1865, 1866). His work was fol- lowed by the comprehensive treatment of Pax & Hoffmann (1914), which included 17 species. Pax & Hoffmann excluded the section Discocleidion Müll.Arg. which differs from Cleidion by the presence of a staminate and pistillate disc (in Cleidion a disc is absent), stipellate and palmatinerved leaves (in Cleidion the leaves are non-stipellate and pinnatinerved), and differences in anther type. -

Expression-Based Genetic/Physical Maps of Single-Nucleotide Polymorphisms Identified by the Cancer Genome Anatomy Project
Downloaded from genome.cshlp.org on October 11, 2021 - Published by Cold Spring Harbor Laboratory Press Resource Expression-based Genetic/Physical Maps of Single-Nucleotide Polymorphisms Identified by the Cancer Genome Anatomy Project Robert Clifford, Michael Edmonson, Ying Hu, Cu Nguyen, Titia Scherpbier, and Kenneth H. Buetow1 Laboratory of Population Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 USA SNPs (Single-Nucleotide Polymorphisms), the most common DNA variant in humans, represent a valuable resource for the genetic analysis of cancer and other illnesses. These markers may be used in a variety of ways to investigate the genetic underpinnings of disease. In gene-based studies, the correlations between allelic variants of genes of interest and particular disease states are assessed. An extensive collection of SNP markers may enable entire molecular pathways regulating cell metabolism, growth, or differentiation to be analyzed by this approach. In addition, high-resolution genetic maps based on SNPs will greatly facilitate linkage analysis and positional cloning. The National Cancer Institute’s CGAP-GAI (Cancer Genome Anatomy Project Genetic Annotation Initiative) group has identified 10,243 SNPs by examining publicly available EST (Expressed Sequence Tag) chromatograms. More than 6800 of these polymorphisms have been placed on expression-based integrated genetic/physical maps. In addition to a set of comprehensive SNP maps, we have produced maps containing single nucleotide polymorphisms in genes expressed in breast, colon, kidney, liver, lung, or prostate tissue. The integrated maps, a SNP search engine, and a Java-based tool for viewing candidate SNPs in the context of EST assemblies can be accessed via the CGAP-GAI web site (http://cgap.nci.nih.gov/GAI/). -

HUMAN GENE MAPPING WORKSHOPS C.1973–C.1991
HUMAN GENE MAPPING WORKSHOPS c.1973–c.1991 The transcript of a Witness Seminar held by the History of Modern Biomedicine Research Group, Queen Mary University of London, on 25 March 2014 Edited by E M Jones and E M Tansey Volume 54 2015 ©The Trustee of the Wellcome Trust, London, 2015 First published by Queen Mary University of London, 2015 The History of Modern Biomedicine Research Group is funded by the Wellcome Trust, which is a registered charity, no. 210183. ISBN 978 1 91019 5031 All volumes are freely available online at www.histmodbiomed.org Please cite as: Jones E M, Tansey E M. (eds) (2015) Human Gene Mapping Workshops c.1973–c.1991. Wellcome Witnesses to Contemporary Medicine, vol. 54. London: Queen Mary University of London. CONTENTS What is a Witness Seminar? v Acknowledgements E M Tansey and E M Jones vii Illustrations and credits ix Abbreviations and ancillary guides xi Introduction Professor Peter Goodfellow xiii Transcript Edited by E M Jones and E M Tansey 1 Appendix 1 Photographs of participants at HGM1, Yale; ‘New Haven Conference 1973: First International Workshop on Human Gene Mapping’ 90 Appendix 2 Photograph of (EMBO) workshop on ‘Cell Hybridization and Somatic Cell Genetics’, 1973 96 Biographical notes 99 References 109 Index 129 Witness Seminars: Meetings and publications 141 WHAT IS A WITNESS SEMINAR? The Witness Seminar is a specialized form of oral history, where several individuals associated with a particular set of circumstances or events are invited to meet together to discuss, debate, and agree or disagree about their memories. The meeting is recorded, transcribed, and edited for publication. -

'Morbid Anatomy' of the Human Genome
Med. Hist. (2014), vol. 58(3), pp. 315–336. c The Author 2014. Published by Cambridge University Press 2014 doi:10.1017/mdh.2014.26 The ‘Morbid Anatomy’ of the Human Genome: Tracing the Observational and Representational Approaches of Postwar Genetics and Biomedicine The William Bynum Prize Essay ANDREW J. HOGAN* Department of History, Humanities Center 225, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA Abstract: This paper explores evolving conceptions and depictions of the human genome among human and medical geneticists during the postwar period. Historians of science and medicine have shown significant interest in the use of informational approaches in postwar genetics, which treat the genome as an expansive digital data set composed of three billion DNA nucleotides. Since the 1950s, however, geneticists have largely interacted with the human genome at the microscopically visible level of chromosomes. Mindful of this, I examine the observational and representational approaches of postwar human and medical genetics. During the 1970s and 1980s, the genome increasingly came to be understood as, at once, a discrete part of the human anatomy and a standardised scientific object. This paper explores the role of influential medical geneticists in recasting the human genome as being a visible, tangible, and legible entity, which was highly relevant to traditional medical thinking and practice. I demonstrate how the human genome was established as an object amenable to laboratory and clinical research, and argue that the observational and representational approaches of postwar medical genetics reflect, more broadly, the interdisciplinary efforts underlying the development of contemporary biomedicine. Keywords: Medical genetics, Biomedicine, Human genome, Chromosomes, Morbid anatomy Introduction In 1982, Victor McKusick, Physician-in-Chief of the Johns Hopkins University School of Medicine, published a commentary entitled, ‘The Human Genome Through the Eyes of a Clinical Geneticist’. -

Homonoia, Lasiococca, Spathiostemon) And
BLUMEA 43 (1998) 131-164 Revisions and phylogenies of Malesian Euphorbiaceae: Subtribe Lasiococcinae (Homonoia, Lasiococca, Spathiostemon) and Clonostylis, Ricinus, and Wetria Peter+C. van Welzen Rijksherbarium / Hortus Botanicus, P. O. Box 9514, 2300 RA Leiden, The Netherlands Summary A cladogram of the subtribe Lasiococcinae (Homonoia, 2 species, Lasiococca , 3 species, and 2 is with the Wetria All three Spathiostemon, species) presented genus as outgroup. taxa are of with Lasiococca and and Homonoia monophyletic groups species Spathiostemonas sistergroups related to both of them. Within Lasiococca, L. comberi and L. malaccensis are probably closest related. The two species of Homonoia are rheophytes, one is restricted to India where it shows two distinct forms, the other species is widespreadfrom India throughout Malesia. Lasiococca is represented by one species in Malesia, L. malaccensis, only known from three localities, ranging from the Malay Peninsula to Sulawesi and the Lesser Sunda Islands. Spathiostemon has two species in Malesia, one is widespread in Malesia, the other one is restricted to part of Peninsular Thailand. known from the Sumatran is Clonostylis, a monotypic genus only type specimen, not synony- mous with Spathiostemon. Clonostylis is seemingly most similar to Mallotus and Macaranga. also is introduced Malesia and is cultivated. It is Ricinus, a monotypic genus, to generally not of the Lasiococcinae. of also for the part The presence phalanged stamens, typical Lasiococcinae, is Ricinus shows and the connective is often a parallel developmentas many more androphores Ricinus classified and it in its subtribe appendaged. cannot readily be retaining present monotypic seems to be the best solution. Wetria shows two species in Malesia. -

The First Genetic Linkage Map for Fraxinus Pennsylvanica and Syntenic Relationships with Four Related Species
Plant Molecular Biology (2019) 99:251–264 https://doi.org/10.1007/s11103-018-0815-9 The first genetic linkage map for Fraxinus pennsylvanica and syntenic relationships with four related species Di Wu1 · Jennifer Koch2 · Mark Coggeshall3,4 · John Carlson1 Received: 13 August 2018 / Accepted: 15 December 2018 / Published online: 2 January 2019 © Springer Nature B.V. 2019 Abstract Key message The genetic linkage map for green ash (Fraxinus pennsylvanica) contains 1201 DNA markers in 23 linkage groups spanning 2008.87cM. The green ash map shows stronger synteny with coffee than tomato. Abstract Green ash (Fraxinus pennsylvanica) is an outcrossing, diploid (2n = 46) hardwood tree species, native to North America. Native ash species in North America are being threatened by the rapid spread of the emerald ash borer (EAB, Agrilus planipennis), an invasive pest from Asia. Green ash, the most widely distributed ash species, is severely affected by EAB infestation, yet few genomic resources for genetic studies and improvement of green ash are available. In this study, a total of 5712 high quality single nucleotide polymorphisms (SNPs) were discovered using a minimum allele frequency of 1% across the entire genome through genotyping-by-sequencing. We also screened hundreds of genomic- and EST-based microsatellite markers (SSRs) from previous de novo assemblies (Staton et al., PLoS ONE 10:e0145031, 2015; Lane et al., BMC Genom 17:702, 2016). A first genetic linkage map of green ash was constructed from 90 individuals in a full-sib family, combining 2719 SNP and 84 SSR segregating markers among the parental maps. The consensus SNP and SSR map contains a total of 1201 markers in 23 linkage groups spanning 2008.87 cM, at an average inter-marker distance of 1.67 cM with a minimum logarithm of odds of 6 and maximum recombination fraction of 0.40. -

Snps) in Genes of the Alcohol Dehydrogenase, Glutathione S-Transferase, and Nicotinamide Adenine Dinucleotide, Reduced (NADH) Ubiquinone Oxidoreductase Families
J Hum Genet (2001) 46:385–407 © Jpn Soc Hum Genet and Springer-Verlag 2001 ORIGINAL ARTICLE Aritoshi Iida · Susumu Saito · Akihiro Sekine Takuya Kitamoto · Yuri Kitamura · Chihiro Mishima Saori Osawa · Kimie Kondo · Satoko Harigae Yusuke Nakamura Catalog of 434 single-nucleotide polymorphisms (SNPs) in genes of the alcohol dehydrogenase, glutathione S-transferase, and nicotinamide adenine dinucleotide, reduced (NADH) ubiquinone oxidoreductase families Received: March 15, 2001 / Accepted: April 6, 2001 Abstract An approach based on development of a large Introduction archive of single-nucleotide polymorphisms (SNPs) throughout the human genome is expected to facilitate large-scale studies to identify genes associated with drug Human genetic variations result from a dynamic process efficacy and side effects, or susceptibility to common over time that includes sudden mutations, random genetic diseases. We have already described collections of SNPs drift, and, in some cases, founder effects. Variations at a present among various genes encoding drug-metabolizing single gene locus are useful as markers of individual risk for enzymes. Here we report SNPs for such enzymes at adverse drug reactions or susceptibility to complex diseases additional loci, including 8 alcohol dehydrogenases, 12 (for reviews, see Risch and Merikangas 1996; Kruglyak glutathione S-transferases, and 18 belonging to the NADH- 1997; McCarthy and Hilfiker 2000). Common types of ubiquinone oxidoreductase family. Among DNA samples sequence variation in the human genome include single- from 48 Japanese volunteers, we identified a total of 434 nucleotide polymorphisms (SNPs), insertion/deletion SNPs at these 38 loci: 27 within coding elements, 52 in 59 polymorphisms, and variations in the number of repeats of flanking regions, five in 59 untranslated regions, 293 in certain motifs (e.g., microsatellites and variable number of introns, 20 in 39 untranslated regions, and 37 in 39 flanking tandem repeat loci). -
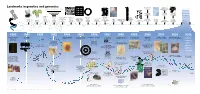
Landmarks in Genetics and Genomics
0 ++- ---+ BC PR • --- -+-t--.--+------ o,ou 1J.T33.? n.e -- -- - 4 Landmarks in genetics and genomics !!!!! U C AG +++j/GA T C US National Research U Phe Tyr Cys International Nucleotide Ser C Council issues report on U Leu stop stop A Sequence Database - stop Trp - G Mapping and Sequencing the U Consortium formed Leu Pro His Arg C Human Genome 0 5' C Gln A 3' G Human U 5' lle Asn Ser STS Markers Thr C A Met Lys Arg A G Muscular-dystrophy Development New strands Sequence-tagged Asp U of yeast artificial Genome G Val Ala Gly C gene identified sites (STS) mapping Glu A chromosome (YAC) G by positional cloning concept established cloning Archibald Garrod Project formulates Alfred Henry Oswald Avery, Colin MacLeod James Watson and Stanley Cohen and Frederick Sanger, First public Desired fragment the concept Sturtevant and Maclyn McCarty Francis Crick Marshall Nirenberg, Herbert Boyer The Belmont Report Allan Maxam discussion strands Gregor Mendel of human makes demonstrate that DNA describe the Har Gobind Khorana and develop on the use of and Walter Gilbert GenBank First human disease gene — for of sequencing First automated First-generation Cystic-fibrosis discovers Rediscovery of inborn errors the first linear is the double-helical Robert Holley determine recombinant human subjects develop DNA-sequencing database Huntington’s disease — is mapped the human The polymerase DNA-sequencing instrument human genetic Human Genome gene identified by laws of genetics Mendel’s work of metabolism map of genes hereditary material structure of DNA the genetic code DNA technology in research is issued methods established with DNA markers genome chain reaction (PCR) is invented developed map developed Organization (HUGO) formed positional cloning 1865 1900 1905 1913 1944 1953 1966 1972 1974 1977 1982 1983 1984 1985 1986 1987 1988 1989 1990 2003 +-+-o-----+---+ 1 I~I "-.■ u ''\. -

Epigenetic Inactivation of a Cluster of Genes Flanking MLH1 in Microsatellite-Unstable Colorectal Cancer
Research Article Epigenetic Inactivation of a Cluster of Genes Flanking MLH1 in Microsatellite-Unstable Colorectal Cancer Megan P. Hitchins,1,5 Vita Ap Lin,1 Andrew Buckle,1 Kayfong Cheong,1 Nimita Halani,1 Su Ku,1 Chau-To Kwok,1 Deborah Packham,1 Catherine M. Suter,3 Alan Meagher,2 Clare Stirzaker,4 Susan Clark,4 Nicholas J. Hawkins,6 and Robyn L. Ward1,5 Departments of 1Medical Oncology and 2Colorectal Surgery, St. Vincent’s Hospital; 3Victor Chang Cardiac Research Centre; 4Garvan Institute of Medical Research, Darlinghurst, Australia; and 5School of Medicine, 6School of Medical Sciences, University of New SouthWales, Sydney, New SouthWales, Australia Abstract genes (1). CpG methylation acts synergistically with repressive histone modifications, such as dimethylation or trimethylation of Biallelic promoter methylation and transcriptional silencing of the MLH1 gene occurs in the majority of sporadic colorectal the histone 3 lysine 9 (H3-K9) residue, to consolidate transcrip- tional silencing (2). CpG island methylation is a common cancers exhibiting microsatellite instability due to defective MLH1 DNA mismatch repair. Long-range epigenetic silencing of epigenetic event in colorectal neoplasia, with promoter contiguous genes has been found on chromosome 2q14 in methylation representing a classic example of this phenomenon. It is well established that biallelic somatic methylation of MLH1 is colorectal cancer. We hypothesized that epigenetic silencing of f MLH1 could occur on a regional scale affecting additional seen in 15% of sporadic colorectal cancers, and these tumors genes within 3p22, rather than as a focal event. We studied the display alterations at microsatellite repeat sequences due to loss of levels of CpG island methylation and expression of multiple DNA mismatchrepair function (3, 4).