Assessment Report on Piper Methysticum G. Forst., Rhizoma Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
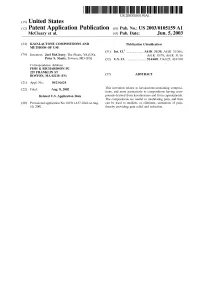
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

Kava (Piper Methysticum) and Its Methysticin Constituents Protect Brain Tissue Against Ischemic Damage in Rodents
5 Refs: Arletti R et al, Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual-behavior of male rats. Psychopharmacology 143(1), 15-19, 1999. Berger F, Handbuch der drogenkunde . Vol 2, Maudrich, Wien, 1950. Martinez M, Les plantas medicinales de Mexico . Cuarta Edicion Botas Mexico , p119, 1959. Tyler VE et al, Pharmacognosy , 9 th edition, Lea & Febiger, Philadelphia, 1988. KAVA ( Piper methysticum ) - A REVIEW The Kava plant (Piper methysticum) is a robust, well-branching and erect perennial shrub belonging to the pepper family (Piperaceae). The botanical origin remains unknown, although it is likely that early Polynesian explorers brought the plant with them from island to island. Numerous varieties of Kava exist, and today it is widely cultivated in several Pacific Island countries both for local use as well as the rapidly growing demand for pharmaceutical preparations. The dried rhizomes (roots) are normally used. The first description to the western world of the ceremonial use of an intoxicating beverage prepared from Kava was made by Captain James Cook following his Pacific voyage in 1768. The drink, prepared as an infusion in an elaborate manner after first chewing the root, is consumed on formal occasions or meetings of village elders and chiefs, as well as in reconciling with enemies and on a more social basis. It remains an important social custom in many Pacific Island countries today. Most of the islands of the Pacific possessed Kava prior to European contact, particularly those encompassed by Polynesia, Melanesia and Micronesia. After drinking the Kava beverage a pleasantly relaxed and sociable state develops, after which a deep and restful sleep occurs. -

Alternative Treatments for Depression and Anxiety
2019 PCB Conference: Strickland Benzodiazepines (BZDs), Herbal and Alternative Treatments for Anxiety & Depression BZD Learning Objectives • List at least three uses for benzodiazepines • Discuss at least two risk factors associated with benzodiazepine prescriptions Craig Strickland, PhD, Owner Biobehavioral Education and Consultation https://sites.google.com/site/bioedcon 1 2 BZD Pharmacokinetics Clinical Uses of BZDs Generic Name Trade Name Rapidity ½ Life Dose (mg) • Treat a variety of anxiety disorders alprazolam Xanax Intermediate Short 0.75-4 • Hypnotics • Muscle relaxants chlordiaze- Librium Intermediate Long 15-100 poxide • To produce anterograde amnesia clonazepam Klonopin Intermediate Long 0.5-4 • Alcohol & other CNS depressant withdrawal • Anti-convulsant therapy diazepam Valium Rapid Long 4-40 triazolam Halcion Intermediate Very short 0.125-0.5 temazepam Restoril Short Short 7.5-30 3 4 1 2019 PCB Conference: Strickland Issues with BZDs Herbal Medication and Alternative Therapies Used in the Treatment of Depression and Anxiety • Addictive potential • Confusion between “anti-anxiety” effects and the “warm-fuzzy) • Large dose ranges • Comparison of BZDs with medications like Buspar, etc. • They work, they work well and they work quickly 5 6 Alternative Tx. Learning Objectives Background Information on herbals: Natural does not necessarily mean “safe” • List several amino acid treatments for depression • Side-effects and adverse reactions • List at least three of the most common herbal – Herbal medications are “drugs” although -

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls
toxins Review Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls 1 2 3, 4, , Marie-Line Reynaert , Denis Dupoiron , Edouard Yeramian y, Laurent Marsollier * y and 1, , Priscille Brodin * y 1 France Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019-UMR8204-CIIL-Center for Infection and Immunity of Lille, F-59000 Lille, France 2 Institut de Cancérologie de l’Ouest Paul Papin, 15 rue André Boquel-49055 Angers, France 3 Unité de Microbiologie Structurale, Institut Pasteur, CNRS, Univ. Paris, F-75015 Paris, France 4 Equipe ATIP AVENIR, CRCINA, INSERM, Univ. Nantes, Univ. Angers, 4 rue Larrey, F-49933 Angers, France * Correspondence: [email protected] (L.M.); [email protected] (P.B.) These three authors contribute equally to this work. y Received: 29 June 2019; Accepted: 3 September 2019; Published: 4 September 2019 Abstract: Pain currently represents the most common symptom for which medical attention is sought by patients. The available treatments have limited effectiveness and significant side-effects. In addition, most often, the duration of analgesia is short. Today, the handling of pain remains a major challenge. One promising alternative for the discovery of novel potent analgesics is to take inspiration from Mother Nature; in this context, the detailed investigation of the intriguing analgesia implemented in Buruli ulcer, an infectious disease caused by the bacterium Mycobacterium ulcerans and characterized by painless ulcerative lesions, seems particularly promising. More precisely, in this disease, the painless skin ulcers are caused by mycolactone, a polyketide lactone exotoxin. In fact, mycolactone exerts a wide range of effects on the host, besides being responsible for analgesia, as it has been shown notably to modulate the immune response or to provoke apoptosis. -

Benzodiazepines: Uses and Risks Charlie Reznikoff, MD Hennepin Healthcare
Benzodiazepines: Uses and Risks Charlie Reznikoff, MD Hennepin healthcare 4/22/2020 Overview benzodiazepines • Examples of benzos and benzo like drugs • Indications for benzos • Pharmacology of benzos • Side effects and contraindications • Benzo withdrawal • Benzo tapers 12/06/2018 Sedative/Hypnotics • Benzodiazepines • Alcohol • Z-drugs (Benzo-like sleeping aids) • Barbiturates • GHB • Propofol • Some inhalants • Gabapentin? Pregabalin? 12/06/2018 Examples of benzodiazepines • Midazolam (Versed) • Triazolam (Halcion) • Alprazolam (Xanax) • Lorazepam (Ativan) • Temazepam (Restoril) • Oxazepam (Serax) • Clonazepam (Klonopin) • Diazepam (Valium) • Chlordiazepoxide (Librium) 4/22/2020 Sedatives: gaba stimulating drugs have incomplete “cross tolerance” 12/06/2018 Effects from sedative (Benzo) use • Euphoria/bliss • Suppresses seizures • Amnesia • Muscle relaxation • Clumsiness, visio-spatial impairment • Sleep inducing • Respiratory suppression • Anxiolysis/disinhibition 12/06/2018 Tolerance to benzo effects? • Effects quickly diminish with repeated use (weeks) • Euphoria/bliss • Suppresses seizures • Effects incompletely diminish with repeated use • Amnesia • Muscle relaxation • Clumsiness, visio-spatial impairment • Seep inducing • Durable effects with repeated use • Respiratory suppression • Anxiolysis/disinhibition 12/06/2018 If you understand this pharmacology you can figure out the rest... • Potency • 1 mg diazepam <<< 1 mg alprazolam • Duration of action • Half life differences • Onset of action • Euphoria, clinical utility in acute -

Is Alcohol Use Related to High Cholesterol in Premenopausal
Research iMedPub Journals Journal of Preventive Medicine 2018 http://www.imedpub.com/ Vol.3 No.1:3 ISSN 2572-5483 DOI: 10.21767/2572-5483.100024 Is Alcohol Use Related To High Cholesterol in Premenopausal Women Aged 40-51 Years Old? Sydnee Homeyer, Jessica L Hartos*, Holli Lueg, Jessica Moore and Patricia Stafford Department of Physician Assistant Studies, University of North Texas Health Science Center, USA *Corresponding author: Jessica L. Hartos, Department of Physician Assistant Studies, University of North Texas Health Science Center, USA, Tel: 817-735-2454; Fax: 817-735-2529; E-mail: [email protected] Received date: November 13, 2017; Accepted date: January 8, 2018; Publication date: January 15, 2018 Citation: Homeyer S, Hartos JL, Lueg H, Moore J, Stafford P, et al. (2018) Is Alcohol Use Related To High Cholesterol In Premenopausal Women Aged 40-51 Years Old? J Prev Med Vol.3 No.3: 3. Copyright: © 2018 Homeyer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Introduction Abstract Increased serum cholesterol levels is a major risk factor for coronary heart disease (CHD) [1], and over seventy-one million Purpose: Alcohol use and cholesterol are related in men and American adults have high cholesterol (27%) [2,3]. In American postmenopausal women but relations between alcohol use women, cholesterol levels have been shown to increase with and cholesterol are unclear for premenopausal women. The age, and nearly 1 in 2 American women has high or borderline purpose of this study was to determine whether alcohol use was related to cholesterol in women aged 40-51 years old. -

E the Effect of Topiramate on Weight Loss in Patients with Type 2 Diabetes
E The effect of topiramate on weight loss in patients with type 2 diabetes RTICL Sedighe Moradi, Scott Reza Jafarian Kerman, Mina Mollabashi A Institute of Endocrinology and Metabolism (Hemmat Campus), Firoozgar Hospital, Tehran University of Medical Sciences, Tehran, Iran Background: Obesity has been associated with several co‑morbidities such as diabetes and increased mortality. In general, the use of medication promotes only a modest weight loss in the range of 2 to 10 kg, usually most effective during the first 6 months of therapy; however, studies have shown positive effects on other risk factors such as blood pressure and serum glucose levels, but there are fewer studies in patients with diabetes. The aim of this study was to assess the effect of topiramate on weight reduction patients with type 2 diabetes. Materials and Methods: This was a 32‑week randomized clinical trial study of 69 subjects during 2008‑2010. RIGINAL Patients, in two treatment groups were given topiramate (39 patients) and Placebo (30 patients) and were subjected to participation in a non‑pharmacologic lifestyle intervention program; which were randomly allocated in our two groups. The percentage change in body O weight and Body Mass Index (BMI) at the end of the study was the primary efficacy endpoint and secondary indicators were changes in blood pressure (BP), proportion of subjects who achieved 5% or 10% weight loss, changes in lipid profile (total cholesterol, low‑density lipoprotein cholesterol, high‑density lipoprotein cholesterol, triglycerides); and changes in glycosylated hemoglobin (HgA1c). Paired samples and independent samples t‑test was used for statistical analysis. -

Central Valley Toxicology Drug List
Chloroform ~F~ Lithium ~A~ Chlorpheniramine Loratadine Famotidine Acebutolol Chlorpromazine Lorazepam Fenoprofen Acetaminophen Cimetidine Loxapine Fentanyl Acetone Citalopram LSD (Lysergide) Fexofenadine 6-mono- Clomipramine acetylmorphine Flecainide ~M~ Clonazepam a-Hydroxyalprazolam Fluconazole Maprotiline Clonidine a-Hydroxytriazolam Flunitrazepam MDA Clorazepate Albuterol Fluoxetine MDMA Clozapine Alprazolam Fluphenazine Medazepam Cocaethylene Amantadine Flurazepam Meperidine Cocaine 7-Aminoflunitrazepam Fluvoxamine Mephobarbital Codeine Amiodarone Fosinopril Meprobamate Conine Amitriptyline Furosemide Mesoridazine Cotinine Amlodipine Methadone Cyanide ~G~ Amobarbital Methanol Cyclobenzaprine Gabapentin Amoxapine d-Methamphetamine Cyclosporine GHB d-Amphetamine l-Methamphetamine Glutethamide l-Amphetamine ~D~ Methapyrilene Guaifenesin Aprobarbital Demoxepam Methaqualone Atenolol Desalkylfurazepam ~H~ Methocarbamol Atropine Desipramine Halazepam Methylphenidate ~B~ Desmethyldoxepin Haloperidol Methyprylon Dextromethoraphan Heroin Metoclopramide Baclofen Diazepam Hexobarbital Metoprolol Barbital Digoxin Hydrocodone Mexiletine Benzoylecgonine Dihydrocodein Hydromorphone Midazolam Benzphetamine Dihydrokevain Hydroxychloroquine Mirtazapine Benztropine Diltiazem Hydroxyzine Morphine (Total/Free) Brodificoum Dimenhydrinate Bromazepam ~N~ Diphenhydramine ~I~ Bupivacaine Nafcillin Disopyramide Ibuprofen Buprenorphine Naloxone Doxapram Imipramine Bupropion Naltrexone Doxazosin Indomethacin Buspirone NAPA Doxepin Isoniazid Butabarbital Naproxen -

Herbal Medicines in Pregnancy and Lactation : an Evidence-Based
00 Prelims 1410 10/25/05 2:13 PM Page i Herbal Medicines in Pregnancy and Lactation An Evidence-Based Approach Edward Mills DPh MSc (Oxon) Director, Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Jean-Jacques Duguoa MSc (cand.) ND Naturopathic Doctor Toronto Western Hospital Assistant Professor Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Dan Perri BScPharm MD MSc Clinical Pharmacology Fellow University of Toronto Toronto, Ontario, Canada Gideon Koren MD FACMT FRCP Director of Motherisk Professor of Medicine, Pediatrics and Pharmacology University of Toronto Toronto, Ontario, Canada With a contribution from Paul Richard Saunders PhD ND DHANP 00 Prelims 1410 10/25/05 2:13 PM Page ii © 2006 Taylor & Francis Medical, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2006 by Taylor & Francis Medical, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN Tel.: ϩ44 (0)20 7017 6000 Fax.: ϩ44 (0)20 7017 6699 E-mail: [email protected] Website: www.tandf.co.uk/medicine All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or trans- mitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. -

NIH Public Access Author Manuscript Pharmacol Ther
NIH Public Access Author Manuscript Pharmacol Ther. Author manuscript; available in PMC 2010 August 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Pharmacol Ther. 2009 August ; 123(2): 239±254. doi:10.1016/j.pharmthera.2009.04.002. Ethnobotany as a Pharmacological Research Tool and Recent Developments in CNS-active Natural Products from Ethnobotanical Sources Will C. McClatcheya,*, Gail B. Mahadyb, Bradley C. Bennettc, Laura Shielsa, and Valentina Savod a Department of Botany, University of Hawaìi at Manoa, Honolulu, HI 96822, U.S.A b Department of Pharmacy Practice, University of Illinois at Chicago, Chicago, IL 60612, U.S.A c Department of Biological Sciences, Florida International University, Miami, FL 33199, U.S.A d Dipartimento di Biologia dì Roma Trè, Viale Marconi, 446, 00146, Rome, Italy Abstract The science of ethnobotany is reviewed in light of its multidisciplinary contributions to natural product research for the development of pharmaceuticals and pharmacological tools. Some of the issues reviewed involve ethical and cultural perspectives of healthcare and medicinal plants. While these are not usually part of the discussion of pharmacology, cultural concerns potentially provide both challenges and insight for field and laboratory researchers. Plant evolutionary issues are also considered as they relate to development of plant chemistry and accessing this through ethnobotanical methods. The discussion includes presentation of a range of CNS-active medicinal plants that have been recently examined in the field, laboratory and/or clinic. Each of these plants is used to illustrate one or more aspects about the valuable roles of ethnobotany in pharmacological research. -

Acute Migraine Treatment
Acute Migraine Treatment Morris Levin, MD Professor of Neurology Director, Headache Center UCSF Department of Neurology San Francisco, CA Mo Levin Disclosures Consulting Royalties Allergan Oxford University Press Supernus Anadem Press Amgen Castle Connolly Med. Publishing Lilly Wiley Blackwell Mo Levin Disclosures Off label uses of medication DHE Antiemetics Zolmitriptan Learning Objectives At the end of the program attendees will be able to 1. List all important options in the acute treatment of migraine 2. Discuss the evidence and guidelines supporting the major migraine acute treatment options 3. Describe potential adverse effects and medication- medication interactions in acute migraine pharmacological treatment Case 27 y/o woman has suffered ever since she can remember from “sick headaches” . Pain is frontal, increases over time and is generally accompanied by nausea and vomiting. She feels depressed. The headache lasts the rest of the day but after sleeping through the night she awakens asymptomatic 1. Diagnosis 2. Severe Headache relief Diagnosis: What do we need to beware of? • Misdiagnosis of primary headache • Secondary causes of headache Red Flags in HA New (recent onset or change in pattern) Effort or Positional Later onset than usual (middle age or later) Meningismus, Febrile AIDS, Cancer or other known Systemic illness - Neurological or psych symptoms or signs Basic principles of Acute Therapy of Headaches • Diagnose properly, including comorbid conditions • Stratify therapy rather than treat in steps • Treat early