Garri, Fufu, Flour and Tapioca) from White and Yellow Cassava Varieties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Igbo Traditional Food System Documented in Four States in Southern Nigeria
Chapter 12 The Igbo traditional food system documented in four states in southern Nigeria . ELIZABETH C. OKEKE, PH.D.1 . HENRIETTA N. ENE-OBONG, PH.D.1 . ANTHONIA O. UZUEGBUNAM, PH.D.2 . ALFRED OZIOKO3,4. SIMON I. UMEH5 . NNAEMEKA CHUKWUONE6 Indigenous Peoples’ food systems 251 Study Area Igboland Area States Ohiya/Ohuhu in Abia State Ubulu-Uku/Alumu in Delta State Lagos Nigeria Figure 12.1 Ezinifite/Aku in Anambra State Ede-Oballa/Ukehe IGBO TERRITORY in Enugu State Participating Communities Data from ESRI Global GIS, 2006. Walter Hitschfield Geographic Information Centre, McGill University Library. 1 Department of 3 Home Science, Bioresources Development 5 Nutrition and Dietetics, and Conservation Department of University of Nigeria, Program, UNN, Crop Science, UNN, Nsukka (UNN), Nigeria Nigeria Nigeria 4 6 2 International Centre Centre for Rural Social Science Unit, School for Ethnomedicine and Development and of General Studies, UNN, Drug Discovery, Cooperatives, UNN, Nigeria Nsukka, Nigeria Nigeria Photographic section >> XXXVI 252 Indigenous Peoples’ food systems | Igbo “Ndi mba ozo na-azu na-anwu n’aguu.” “People who depend on foreign food eventually die of hunger.” Igbo saying Abstract Introduction Traditional food systems play significant roles in maintaining the well-being and health of Indigenous Peoples. Yet, evidence Overall description of research area abounds showing that the traditional food base and knowledge of Indigenous Peoples are being eroded. This has resulted in the use of fewer species, decreased dietary diversity due wo communities were randomly to household food insecurity and consequently poor health sampled in each of four states: status. A documentation of the traditional food system of the Igbo culture area of Nigeria included food uses, nutritional Ohiya/Ohuhu in Abia State, value and contribution to nutrient intake, and was conducted Ezinifite/Aku in Anambra State, in four randomly selected states in which the Igbo reside. -

Malawian Food Composition Table 2019
GOVERNMENT OF MALAWI MALAWIAN FOOD COMPOSITION TABLE 2019 February 2020 FOREWORD Adequate nutrition throughout the lifecycle is the As one way of ensuring adequate nutrition and centerpiece for every individual’s physical and improved standards of locally-processed foods intellectual development since nutrition is a key for the general population, the GoM, with support determinant of one’s intellectual performance, from the USAID Feed the Future Innovation Lab academic and professional achievements, and overall for Nutrition at the Friedman School of Nutrition work productivity. Because nutrition is fundamental Science and Policy, Tufts University, South African for socioeconomic growth and development of Food Data System (SAFOODS) of the South African the country, the Government of Malawi (GoM) has Medical Research Council, and Lilongwe University identified good nutrition as a key priority in its national of Agriculture & Natural Resources (LUANAR) and development agenda articulated in the Malawi financial support from USAID, Malawi embarked Growth Development Strategy III. To operationalize to develop a food composition database. The the Malawi Growth and Development Strategy III, the Malawian Food Composition Database (MFCDB) GoM developed a comprehensive National Multi- and subsequent publication of the information from Sector Policy and Strategic Plan 2018-2022, which the Database, into the Malawian Food Composition incorporates emerging issues such as diet-related, Table (MFCT) describe the nutritive value of locally- noncommunicable diseases. Additionally, the GoM produced and imported foods that are available is developing the food and nutrition legislation to in Malawi. Country-specific food composition support implementation of the National Multi-Sector databases and tables are essential tools for assessing Nutrition Policy (NMNP). -

Functional and Pasting Properties of Products Of
1 Journal of Agriculture and Food Sciences Onyeneke, Esther-Ben Volume 17 Number 1, April 2019 pp 1 - 17 . FUNCTIONAL AND PASTING PROPERTIES OF PRODUCTS OF WHITE AND YELLOW CASSAVA Onyeneke, Esther-Ben Department of Nutrition and Dietetics, Faculty of Health Science, Imo State University Email: estyninika@ gmail.com ABSTRACT This study evaluated the pasting properties of selected products; fufu, garri and tapioca made from yellow cassava (Manihot utilisima Crantz) and white cassava (Manihot dulcis Crantz) products. The cassava products were obtained from the National Root Crops Research Institute, Umudike, Abia State and was processed in food science and technology and nutrition and dietetics laborstory, Imo state university. The functional and pasting result showed that among the studied finished cassava products, the fermented cassava flour had the highest water absorption capacity (WAC) 4.4g/g as compared to values of 3.75g/g for garri and 2.85g/g for tapioca.The water absorption capacities of the white cassava products were in the range of 2.79±0.01to 4.34±0.14g/g with white cassava tapioca having the least value (2.79±0.0g/g) and the white cassava flour having the highest value (4.34±0.14). Some significant differences (p<0.05) existed between the water absorption capacities value of the match pairs (similar products) of white and yellow cassava varieties, with the exception of the garri samples. The oil absorption capacities (OAC) of the yellow cassava products were in the range of 1.94±0.03 to 2.91±0.01g/g with yellow cassava tapioca having the least value (1.94±0.03g/g) e and the yellow cassava flour having the highest value (2.91±0.01g/g). -

Purple Hibiscus
1 A GLOSSARY OF IGBO WORDS, NAMES AND PHRASES Taken from the text: Purple Hibiscus by Chimamanda Ngozi Adichie Appendix A: Catholic Terms Appendix B: Pidgin English Compiled & Translated for the NW School by: Eze Anamelechi March 2009 A Abuja: Capital of Nigeria—Federal capital territory modeled after Washington, D.C. (p. 132) “Abumonye n'uwa, onyekambu n'uwa”: “Am I who in the world, who am I in this life?”‖ (p. 276) Adamu: Arabic/Islamic name for Adam, and thus very popular among Muslim Hausas of northern Nigeria. (p. 103) Ade Coker: Ade (ah-DEH) Yoruba male name meaning "crown" or "royal one." Lagosians are known to adopt foreign names (i.e. Coker) Agbogho: short for Agboghobia meaning young lady, maiden (p. 64) Agwonatumbe: "The snake that strikes the tortoise" (i.e. despite the shell/shield)—the name of a masquerade at Aro festival (p. 86) Aja: "sand" or the ritual of "appeasing an oracle" (p. 143) Akamu: Pap made from corn; like English custard made from corn starch; a common and standard accompaniment to Nigerian breakfasts (p. 41) Akara: Bean cake/Pea fritters made from fried ground black-eyed pea paste. A staple Nigerian veggie burger (p. 148) Aku na efe: Aku is flying (p. 218) Aku: Aku are winged termites most common during the rainy season when they swarm; also means "wealth." Akwam ozu: Funeral/grief ritual or send-off ceremonies for the dead. (p. 203) Amaka (f): Short form of female name Chiamaka meaning "God is beautiful" (p. 78) Amaka ka?: "Amaka say?" or guess? (p. -

Original Research Article Nutritional and Anti–Nutritional Evaluation of Garri Processed by Traditional and Instant Mechanical
Original Research Article Nutritional and Anti–Nutritional Evaluation of Garri Processed by Traditional and Instant Mechanical Methods ABSTRACT Aim : The aim of this study is to evaluate the nutritional and anti-nutritional composition of garri processed by traditional and instant mechanical methods. Place and Duration of Study : This study was carried out in Achara, Uturu and analyses were done at the Biochemistry and Central Laboratories of Gregory University, Uturu, Abia State between March and July, 2017. Methods : Cassava was harvested and processed in Achara area of Uturu, Abia State. For garri processed by instant mechanical method, cassava was grated and dewatered using hydraulic press and were roasted (fried) within 24 hours of harvest. For garri processed by traditional method, the grated garri was allowed to stay for 24 hours in the sack before dewatering using sticks. The dewatering process took 3 days before roasting. The nutritional and anti-nutritional composition of raw cassava mash, garri processed by traditional and instant mechanical methods were evaluated using standard methods. Results : The result of the analysis showed that garri processed by traditional method was higher in most of the nutritional factors but lower in all of the anti-nutritional factors investigated when compared with those of garri processed by instant mechanical method and raw cassava mash. Garri processed by traditional method was significantly higher in vitamin A but lower in vitamin C when compared with garri processed by instant mechanical method at p<0.05. Garri with palm oil has its cyanogenic glucoside significantly reduced when compared with garri without palm oil at p<0.05. -
Vegetarian Menu
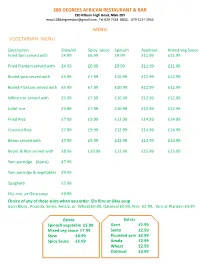
280 DEGREES AFRICAN RESTAURANT & BAR 280 Kilburn High Road, NW6 2BY email:[email protected], Tel:020 7328 8832, 079 5137 5954 MENU VEGETARIAN MENU Description Stew/nil Spicy sauce Spinach Ayamase Mixed Veg Sauce Fried Yam served with £4.99 £6.99 £9.99 £11.99 £11.99 Fried Plantain served with £4.99 £6.99 £9.99 £11.99 £11.99 Boiled yam served with £5.99 £7.99 £10.99 £12.99 £12.99 Boiled Plantain served with £5.99 £7.99 £10.99 £12.99 £12.99 White rice served with £5.99 £7.99 £10.99 £12.99 £12.99 Jollof rice £5.99 £7.99 £10.99 £12.99 £12.99 Fried Rice £7.99 £9.99 £12.99 £14.99 £14.99 Coconut Rice £7.99 £9.99 £12.99 £14.99 £14.99 Beans served with £7.99 £9.99 £12.99 £14.99 £14.99 Beans & Rice served with £8.99 £10.99 £13.99 £15.99 £15.99 Yam porridge (Asaro) £7.99 Yam porridge & vegetables £9.99 Spaghetti £5.99 Efo -riro or Okra soup £9.99 Choice of any of these sides when you order Efo Riro or Okra soup Garri (Eba) , Poundo, Semo, Amala, or Wheat £0.00, Oatmeal £0.99, Rice £2.99, Yam or Plantain £3.99 Extras Extras Spinach vegetable £5.99 Garri £2.99 Mixed veg sauce £7.99 Semo £2.99 Stew £4.99 Pounded yam £2.99 Spicy Sauce £5.99 Amala £2.99 Wheat £2.99 Oatmeal £3.99 LIGHT DISHES (STÄRTERS) NIBBLES (PER PORTION) Akara (Pre - booked) £2.50, Puff – Puff £2.50, Meat Pie £2.50, Chicken Pie £2.50, Fish Pie £2.50 Fried Beef £5.99 Fried Goat Meat £7.99 AFRICAN SPICY PEPPER SOUP (All pepper soup dishes are made from a blend of spicy African pepper, herbs & spices) Assorted Goat meat Pepper soup £9.99 Chicken Pepper soup £9.99 Catfish Pepper -

Kola Lounge Menu-Back-V15
E N T I T H C U A A F R I C CHECK OUT OURA WINE LIST ON N THE BACK C A R I B B 32523 Northwestern Hwy | Farmington Hills, MI 48334 E A N C S I C (248) 932-KOLA (5652) U I S I N E / M U Email [email protected] BEVERAGES ENTRÉES SOFT DRINKS 3.5 TRADITIONAL VEGETABLE SOUPS Coke • Diet Coke • Sprite Lemonade Served with your choice of: RICE: White/Jollof/Coconut/Fried FRUIT JUICES 3.5 FUFU: Pounded Yam (Yam Flour) Orange • Cranberry • Pineapple • Apple • Coconut Water Garri (Ground Cassava) Meats: Beef, Fish or Chicken MALTA 3.5 Stewed Oxtail: Add $6 Stewed Goat: Add $2.5 KOLA CHAMPAGNE 3.5 EGUSI 17.75 BOTTLED WATER 2.5 Ground melon seeds steamed with spinach, dried fish and palm oil BOTTLED PALM JUICE EDIKANG IKONG 17.75 10 Spinach, dried fish, kale, spices and palm oil OKRA 17.75 Whipped okra with blend of tomatoes,onions, palm oil, dried fish APPETIZERS & spices MOI MOI RICE DISHES 6.75 Served with your choice of: Steamed bean pudding made from blackeyed peas, onions, fresh ground Meats: Beef, Fish, Chicken, Curry Chicken of Jerk Chicken peppers, crayfish seasoning and boiled egg Stewed Oxtail: Add $6 MEAT PIE Stewed Goat: Add $2.5 4.75 Plantain add $3.5 Pie with a filling of seasoned beef, potatoes and onions WHITE RICE PLANTAIN (STEW) 7.75 15.25 JOLLOF RICE 16.75 BEEF OR CHICKEN SUYA 13.75 Steamed rice with a blend of tomatoes, onions, red bell peppers Thinly sliced, grilled beef or chicken seasoned with yaji spice, onions and and spices tomatoes FRIED RICE 16.75 KOLA CHICKEN WINGS (JERK 13.25) 11.75 Steamed rice with blend of vegetables -

Nigerian Traditional Food System and Nutrition Security
NIGERIANNIGERIAN TRADITIONALTRADITIONAL FOODFOOD SYSTEMSYSTEM ANDAND NUTRITIONNUTRITION SECURITYSECURITY PROF. IGNATIUS ONIMAWO (PhD) BIOCHEMISTRY DEPARTMENT AMBROSE ALLI UNIVERSITY, EKPOMA, NIGERIA [email protected] President Nutrition Society of Nigeria International Scientific Symposium BIODIVERSITY AND SUSTAINABLE DIETS United Against Hunger 3-5 November 2010. INTRODUCTIONINTRODUCTION • Traditional food systems– refer to the human managed biophysical systems that are involved in the production, distribution and consumption of food in a particular environment. • Food systems are a natural locus for improving nutrition security in societies because agriculture is the primary employment sector for the ultra poor and because food consumes a very large share of the expenditures of the ultra poor. • The causal mechanisms underpinning the poverty trap are clearly rooted in the food system that guides their production, exchange, consumption and investment behaviours. • The most basic thing we know is that ill health, malnutrition and ultra poverty are mutually reinforcing states. • The links are multidirectional. Low real incomes are the primary cause of chronic and acute hunger • Even when food availability is adequate – low incomes impede access to sufficient and appropriate food to maintain a healthy lifestyle. • Undernutrition, including micronutrient deficiencies, is the leading risk factor for disease and death worldwide, accounting for over half the disease burden in low income countries. • Undernutrition also impedes cognitive and physical development, thereby depressing educational attainment and adult earnings. • Disease, in turn, impedes the uptake of scarce nutrients, aggravating hunger and micronutrient malnutrition problems and hurting labor productivity and earnings. • Food systems are the natural locus for developing an integrated strategy for addressing hunger, ill health and poverty jointly and thus assuring nutrition security. -

The Resistant Starch Content of Some Cassava Based Nigerian Foods
HOSTED BY Available online at www.sciencedirect.com Nigerian Food Journal 33 (2015) 29–34 www.elsevier.com/locate/nifoj The resistant starch content of some cassava based Nigerian foods Frank C. Ogbon, Edith N. Okafor Department of Applied Microbiology & Brewing, Nnamdi Azikiwe University, Awka, Nigeria Available online 23 May 2015 Abstract The resistant starch (RS) content of some Nigerian cassava varieties and staples, “fufu”, “garri” and “abacha” processed from them were determined. Tubers of six varieties studied contained different concentrations of resistant starch, ranging from 5.70% in TMS 4(2)1425 to 7.07% in the TMS 30,572. Processing using traditional methods reduced the RS content in all cassava based foods compared with tubers from which they were processed. RS concentration was reduced by an average of 70.4% in “fufu”, 52.8% in “garri” and 35.85% in “abacha” for the four varieties of cassava tested. Cassava processing steps involving fermentation were responsible for the major reductions in concentration of RS. Steps involving cooking or frying, resulted in increase in concentrations of RS relative to other processing methods. Modifications of traditional methods of processing such as the addition of bitter leaf during retting or the addition of oil to mash during dewatering of “garri” affected RS concentrations in foods studied. Results of this work suggest that manipulation of processing methods and conditions employed during cassava processing can be used to improve RS concentration in cassava based foods, thus making them more functional. & 2015 Association of Vice-Chancellors of Nigerian Universities. Production and hosting by Elsevier B.V. -

Omslag Report V2
The aflatoxin situation in Africa Systematic literature review RIKILT report 2018.010 The aflatoxin situation in Africa Systematic literature review Nathan Meijer 1, Gijs Kleter 1, Rosa Amalia Safitri 1, Monique de Nijs 1, Marie-Luise Rau 2, Ria Derkx 3, Joke Webbink 3, Marijn Post 3, Yuca Waarts 2, Ine van der Fels-Klerx 1 1 RIKILT Wageningen University & Research 2 Wageningen Economic Research 3 Wageningen University & Research - Library This research has been carried out by Wageningen University & Research and financed by Partnership for Aflatoxin Control in Africa (PACA) through funds made available to PACA by the Bill and Melinda Gates Foundation and Mars, Incorporated (project number 1277360301). PACA acknowledges the contribution of the Technical Centre for Agricultural and Rural Cooperation (CTA) in producing this report which is a follow up to the CTA/PACA 2016 Working Paper “Improving the evidence base on aflatoxin contamination and exposure in Africa” written by Sheila Okoth. Wageningen, December 2018 RIKILT report 2018.010 RIKILT report 2018.010 | 1 Project number: 1277360301 Project title: The aflatoxin situation in Africa Project leader: Nathan Meijer © 2018 African Union Commission / PACA. This study was financed by Partnership for Aflatoxin Control in Africa (PACA) through funds made available to PACA by the Bill and Melinda Gates Foundation and Mars, Incorporated. PACA acknowledges the contribution of the Technical Centre for Agricultural and Rural Cooperation (CTA) in producing this report which is a follow up to the CTA/PACA 2016 Working Paper “Improving the evidence base on aflatoxin contamination and exposure in Africa” written by Sheila Okoth. This report is published by RIKILT Wageningen University & Research, institute within the legal entity Wageningen Research Foundation with the copyright holder’s permission. -

With Co-Morbid Condition on Diabetes Mellitus
© 2021 JETIR February 2021, Volume 8, Issue 2 www.jetir.org (ISSN-2349-5162) A review Comparative Interracial prevalence analysis of COVID-19 patients: With co-morbid condition On Diabetes Mellitus 1Mustapha Ibrahim Abdullahi*, 1Rakesh Das,Smranjeet Kaur, Department of Pharmacology, School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab Abstract: Background: The main objective of this study is to identify the impact of traditional dietary factors of both continents (Africa & India) to control COVID-19 by remarkable reductions in co morbid condition responsible for the pandemic situation. The reduction of risk factors by decreasing of morbidity and mortality of one continent will be the inspiration points of other continents, also in future for these types of pandemic infection. Methodology: As immunity is the fundamental factors to arrest the disastrous effects of this deadly virus, so more implicitly has to be focused on that with best observation data investigation. Although the assessment of the comparative interracial prevalence of COVID patients on support of comorbid condition is sharply based on the observation of various relevant data and its statistical analysis. Conclusion: Still work of observation is undergoing, the comparative data reveals the traditional diet associated with Diabetes Mellitus, a pandemic cause of COVID-19. Key-words: COVID-19, Traditional dietary components, Diabetes Mellitus, Interracial comparative data. Observational studies between Indian and African (Nigerian) population regarding COVID-19 viabilities shows remarkable diversities. These differences are purely based on the traditional dietary stimulated genetic make-up which reduces the Diabetes Mellitus incidence cases in Nigerian population comparatively. Incidence of COVID-19 could easily be controlled after following remarkable dietary habitat. -

The Case for Indigenous West African Food Culture; BREDA Series; Vol.:9
Optical Character Recognition (OCR) document. WARNING! Spelling errors might subsist. In order to access to the original document in image form, click on "Original" button on 1st page. THE CASE FOR INDIGENOUS WEST AFRICAN FOOD CULTURE by Ifeyironwa Francisca Smith (Ph.D) DAKAR, 1995 Optical Character Recognition (OCR) document. WARNING! Spelling errors might subsist. In order to access to the original document in image form, click on "Original" button on 1st page. This research-based publication is, necessarily, part of the gospel of cultural industries which, for decades, UNESCO has Iaboured to spread in the world in general and, since 1985 through BREDA, in Africa in particular. Presented on 19 May 1994 as one of the events to celebrate World Culture Day in cooperation with the Government of the Republic of Senegal, its message is clear and heartening: West Africa could be self-sufficient in food production; traditional food is healthier and cheaper than imported products. In the wider world (MONDIACULT in UNESCO’s vocabulary) Mrs (Dr) Smith’s research discovery illustrates a truth which UNESCO has never failed to reiterate time and again - the constance of cultural Self-Affirmation in ALL human endeavors. In clear terms neither globalisation of industrial production nor internalisation of exchange of goods should result in a uniformation or universalisation that would negate or neutralise the self-identity of peoples expressible through their distinct, unique creative and inventive genius. Nearer home (AFRICULT) the gospel has reached the ears of African Heads of State and Governments who in their 1992 Summit in Senegal formally acknowledged the umbilical cord between Culture and Development and in particular the cultural industries to which a truly endogeneous the social, economic and cultural growth and development of the continent is inexorably linked.