The Effects of Fruit Availability and Habitat Disturbance on an Assemblage of Sumatran Hornbills
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Missionary Perspectives and Experiences of 19Th and Early 20Th Century Droughts
1 2 “Everything is scorched by the burning sun”: Missionary perspectives and 3 experiences of 19th and early 20th century droughts in semi-arid central 4 Namibia 5 6 Stefan Grab1, Tizian Zumthurm,2,3 7 8 1 School of Geography, Archaeology and Environmental Studies, University of the 9 Witwatersrand, South Africa 10 2 Institute of the History of Medicine, University of Bern, Switzerland 11 3 Centre for African Studies, University of Basel, Switzerland 12 13 Correspondence to: Stefan Grab ([email protected]) 14 15 Abstract. Limited research has focussed on historical droughts during the pre-instrumental 16 weather-recording period in semi-arid to arid human-inhabited environments. Here we describe 17 the unique nature of droughts over semi-arid central Namibia (southern Africa) between 1850 18 and 1920. More particularly, our intention is to establish temporal shifts of influence and 19 impact that historical droughts had on society and the environment during this period. This is 20 achieved through scrutinizing documentary records sourced from a variety of archives and 21 libraries. The primary source of information comes from missionary diaries, letters and reports. 22 These missionaries were based at a variety of stations across the central Namibian region and 23 thus collectively provide insight to sub-regional (or site specific) differences in hydro- 24 meteorological conditions, and drought impacts and responses. Earliest instrumental rainfall 25 records (1891-1913) from several missionary stations or settlements are used to quantify hydro- 26 meteorological conditions and compare with documentary sources. The work demonstrates 27 strong-sub-regional contrasts in drought conditions during some given drought events and the 28 dire implications of failed rain seasons, the consequences of which lasted many months to 29 several years. -

Literariness.Org-Mareike-Jenner-Auth
Crime Files Series General Editor: Clive Bloom Since its invention in the nineteenth century, detective fiction has never been more pop- ular. In novels, short stories, films, radio, television and now in computer games, private detectives and psychopaths, prim poisoners and overworked cops, tommy gun gangsters and cocaine criminals are the very stuff of modern imagination, and their creators one mainstay of popular consciousness. Crime Files is a ground-breaking series offering scholars, students and discerning readers a comprehensive set of guides to the world of crime and detective fiction. Every aspect of crime writing, detective fiction, gangster movie, true-crime exposé, police procedural and post-colonial investigation is explored through clear and informative texts offering comprehensive coverage and theoretical sophistication. Titles include: Maurizio Ascari A COUNTER-HISTORY OF CRIME FICTION Supernatural, Gothic, Sensational Pamela Bedore DIME NOVELS AND THE ROOTS OF AMERICAN DETECTIVE FICTION Hans Bertens and Theo D’haen CONTEMPORARY AMERICAN CRIME FICTION Anita Biressi CRIME, FEAR AND THE LAW IN TRUE CRIME STORIES Clare Clarke LATE VICTORIAN CRIME FICTION IN THE SHADOWS OF SHERLOCK Paul Cobley THE AMERICAN THRILLER Generic Innovation and Social Change in the 1970s Michael Cook NARRATIVES OF ENCLOSURE IN DETECTIVE FICTION The Locked Room Mystery Michael Cook DETECTIVE FICTION AND THE GHOST STORY The Haunted Text Barry Forshaw DEATH IN A COLD CLIMATE A Guide to Scandinavian Crime Fiction Barry Forshaw BRITISH CRIME FILM Subverting -

How Climate Change Scorched the Nation in 2012
HOW CLIMATE CHANGE SCORCHED THE NATION IN 2012 NatioNal Wildlife federatioN i. Intro- ductioN Photo: Flickr/woodleywonderworks is climate change ruining our summers? It is certainly altering them in dramatic ways, and rarely for the better. The summer of 2012 has been full of extreme weather events connected to climate change. Heat records have been broken across the country, drought conditions forced the United States Department of Agriculture (USDA) to make the largest disaster declaration in U.S. history, and wildfires have raged throughout the West. New research by world-renowned climate scientist James Hansen con- firms that the increasingly common extreme weather events across the country, like record heat waves and drought, are linked to climate change.1 This report examines those climate change impacts whose harm is acutely felt in the summer. Heat waves; warming rivers, lakes, and streams; floods; drought; wildfires; and insect and pest infestations are problems we are dealing with this summer and what we are likely to face in future summers. As of August 23rd, 7 MILLION 2/3 of the country has experienced drought this acres of wildlife habitat and communities summer, much of it labeled “severe” 4 have burned in wildfires2 40 20 60 More than July’s average0 continental U.S. temperature 80 -20 113 MILLION 77.6°F100 people in the U.S. were in areas under 3.3°F above-40 the 20th-century average. 3 5 extreme heat advisories as of June 29 This was the-60 warmest month120 on record 0F NatioNal Wildlife federatioN Unfortunately, hot summers like this will occur much more frequently in years ahead. -
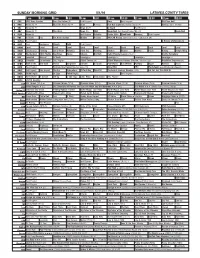
Sunday Morning Grid 5/1/16 Latimes.Com/Tv Times
SUNDAY MORNING GRID 5/1/16 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Boss Paid Program PGA Tour Golf 4 NBC News (N) Å Meet the Press (N) Å News Rescue Red Bull Signature Series (Taped) Å Hockey: Blues at Stars 5 CW News (N) Å News (N) Å In Touch Paid Program 7 ABC News (N) Å This Week News (N) NBA Basketball First Round: Teams TBA. (N) Basketball 9 KCAL News (N) Joel Osteen Schuller Pastor Mike Woodlands Amazing Paid Program 11 FOX In Touch Paid Fox News Sunday Midday Prerace NASCAR Racing Sprint Cup Series: GEICO 500. (N) 13 MyNet Paid Program A History of Violence (R) 18 KSCI Paid Hormones Church Faith Paid Program 22 KWHY Local Local Local Local Local Local Local Local Local Local Local Local 24 KVCR Landscapes Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexico Martha Pépin Baking Simply Ming 28 KCET Wunderkind 1001 Nights Bug Bites Space Edisons Biz Kid$ Celtic Thunder Legacy (TVG) Å Soulful Symphony 30 ION Jeremiah Youssef In Touch Leverage Å Leverage Å Leverage Å Leverage Å 34 KMEX Conexión En contacto Paid Program Fútbol Central (N) Fútbol Mexicano Primera División: Toluca vs Azul República Deportiva (N) 40 KTBN Walk in the Win Walk Prince Carpenter Schuller In Touch PowerPoint It Is Written Pathway Super Kelinda Jesse 46 KFTR Paid Program Formula One Racing Russian Grand Prix. -

Effects of a Prescribed Fire on Oak Woodland Stand Structure1
Effects of a Prescribed Fire on Oak Woodland Stand Structure1 Danny L. Fry2 Abstract Fire damage and tree characteristics of mixed deciduous oak woodlands were recorded after a prescription burn in the summer of 1999 on Mt. Hamilton Range, Santa Clara County, California. Trees were tagged and monitored to determine the effects of fire intensity on damage, recovery and survivorship. Fire-caused mortality was low; 2-year post-burn survey indicates that only three oaks have died from the low intensity ground fire. Using ANOVA, there was an overall significant difference for percent tree crown scorched and bole char height between plots, but not between tree-size classes. Using logistic regression, tree diameter and aspect predicted crown resprouting. Crown damage was also a significant predictor of resprouting with the likelihood increasing with percent scorched. Both valley and blue oaks produced crown resprouts on trees with 100 percent of their crown scorched. Although overall tree damage was low, crown resprouts developed on 80 percent of the trees and were found as shortly as two weeks after the fire. Stand structural characteristics have not been altered substantially by the event. Long term monitoring of fire effects will provide information on what changes fire causes to stand structure, its possible usefulness as a management tool, and how it should be applied to the landscape to achieve management objectives. Introduction Numerous studies have focused on the effects of human land use practices on oak woodland stand structure and regeneration. Studies examining stand structure in oak woodlands have shown either persistence or strong recruitment following fire (McClaran and Bartolome 1989, Mensing 1992). -

The Forest Resiliency Burning Pilot Project
R E S O U C The Forest Resiliency Burning Pilot Project December 2018 N A T U R L The Forest Resiliency Burning Pilot Project Report to the Legislature December 2018 Prepared by Washington State Department of Natural Resources and Washington Prescribed Fire Council Cover photo by © John Marshall. ii Executive Summary More than 100 years of fire suppression and land management practices have severely degraded Eastern Washington’s fire-adapted dry forests. Without the regular, low-intensity fires that created their open stand structure and resiliency, tree density has increased and brush and dead fuels have accumulated in the understory. The impact of these changes in combination with longer fire seasons have contributed to back-to-back record-breaking wildfire years, millions spent in firefighting resources and recovery, danger to our communities, and millions of acres of severely burned forest. Forest resiliency burning, also called prescribed fire or controlled burning, returns fire as an essential ecological process to these forests and is an effective tool for reducing fuels and associated risk of severe fires. Forest experts have identified 2.7 million acres of Central and Eastern Washington forests in need of restoration (Haugo et al. 2015). The agency’s 20-year Forest Health Strategic Plan addresses the need to increase the pace and scale of forest restoration treatments, which includes the use of prescribed fire. Successful implementation of prescribed fire in dry forest ecosystems faces a number of challenges, primarily unfavorable weather conditions, smoke management regulations, and some public opposition. Recognizing these challenges, the urgent need for large-scale forest restoration, and the usefulness and benefits of prescribed fire, the Legislature passed Engrossed Substitute House Bill (ESHB) 2928. -

MAGAZINE ® ISSUE 6 Where Everyone Goes for Scripts and Writers™
DECEMBER VOLUME 17 2017 MAGAZINE ® ISSUE 6 Where everyone goes for scripts and writers™ Inside the Mind of a Thriller Writer PAGE 8 Q&A with Producer Lauren de Normandie of Status Media & Entertainment PAGE 14 FIND YOUR NEXT SCRIPT HERE! CONTENTS Contest/Festival Winners 4 Feature Scripts – FIND YOUR Grouped by Genre SCRIPTS FAST 5 ON INKTIP! Inside the Mind of a Thriller Writer 8 INKTIP OFFERS: Q&A with Producer Lauren • Listings of Scripts and Writers Updated Daily de Normandie of Status Media • Mandates Catered to Your Needs & Entertainment • Newsletters of the Latest Scripts and Writers 14 • Personalized Customer Service • Comprehensive Film Commissions Directory Scripts Represented by Agents/Managers 40 Teleplays 43 You will find what you need on InkTip Sign up at InkTip.com! Or call 818-951-8811. Note: For your protection, writers are required to sign a comprehensive release form before they place their scripts on our site. 3 WHAT PEOPLE SAY ABOUT INKTIP WRITERS “[InkTip] was the resource that connected “Without InkTip, I wouldn’t be a produced a director/producer with my screenplay screenwriter. I’d like to think I’d have – and quite quickly. I HAVE BEEN gotten there eventually, but INKTIP ABSOLUTELY DELIGHTED CERTAINLY MADE IT HAPPEN WITH THE SUPPORT AND FASTER … InkTip puts screenwriters into OPPORTUNITIES I’ve gotten through contact with working producers.” being associated with InkTip.” – ANN KIMBROUGH, GOOD KID/BAD KID – DENNIS BUSH, LOVE OR WHATEVER “InkTip gave me the access that I needed “There is nobody out there doing more to directors that I BELIEVE ARE for writers than InkTip – nobody. -

British Scorched Earth and Concentration Camp Policies
72 THE BRITISH SCORCHED EARTH AND CONCENTRATION CAMP POLICIES IN THE 1 POTCHEFSTROOM REGION, 1899–1902 Prof GN van den Bergh Research Associate, North-West University Abstract The continued military resistance of the Republics after the occupation of Bloemfontein and Pretoria and exaggerated by the advent of guerrilla tactics frustrated the British High Command. In the case of the Potchefstroom region, British aggravation came to focus on the successful resurgence of the Potchefstroom Commando, under Gen. Petrus Liebenberg, swelled by surrendered burghers from the Gatsrand again taking up arms. A succession of proclamations of increasing severity were directed at civilians for lending support to commandos had no effect on either the growth or success of Liebenberg’s commando. His basis for operations was the Gatsrand from where he disrupted British supply communications. He was involved in British evacuations of the town in July and August 1900 and in assisting De Wet in escaping British pursuit in August 1900. British policy came to revolve around denying Liebenberg use of the abundant food supplies in the Gatsrand by applying a scorched earth policy there and in the adjacent Mooi River basin. This occurred in conjuncture with the brief second and permanent third occupation of Potchefstroom. The subsequent establishment of garrisons there gave rise to the systematic destruction of the Gatsrand agricultural infrastructure. To deny further use of the region by commandos it was depopulated. In consequence, the first and largest concentration camp in the Transvaal was established in Potchefstroom. The policies succeeded in dispelling Liebenberg from the region. Introduction Two of the most controversial aspects of the Anglo Boer War are the closely related British scorched earth and concentration camp policies. -

Numb3rs Episode Guide Episodes 001–118
Numb3rs Episode Guide Episodes 001–118 Last episode aired Friday March 12, 2010 www.cbs.com c c 2010 www.tv.com c 2010 www.cbs.com c 2010 www.redhawke.org c 2010 vitemo.com The summaries and recaps of all the Numb3rs episodes were downloaded from http://www.tv.com and http://www. cbs.com and http://www.redhawke.org and http://vitemo.com and processed through a perl program to transform them in a LATEX file, for pretty printing. So, do not blame me for errors in the text ^¨ This booklet was LATEXed on June 28, 2017 by footstep11 with create_eps_guide v0.59 Contents Season 1 1 1 Pilot ...............................................3 2 Uncertainty Principle . .5 3 Vector ..............................................7 4 Structural Corruption . .9 5 Prime Suspect . 11 6 Sabotage . 13 7 Counterfeit Reality . 15 8 Identity Crisis . 17 9 Sniper Zero . 19 10 Dirty Bomb . 21 11 Sacrifice . 23 12 Noisy Edge . 25 13 Man Hunt . 27 Season 2 29 1 Judgment Call . 31 2 Bettor or Worse . 33 3 Obsession . 37 4 Calculated Risk . 39 5 Assassin . 41 6 Soft Target . 43 7 Convergence . 45 8 In Plain Sight . 47 9 Toxin............................................... 49 10 Bones of Contention . 51 11 Scorched . 53 12 TheOG ............................................. 55 13 Double Down . 57 14 Harvest . 59 15 The Running Man . 61 16 Protest . 63 17 Mind Games . 65 18 All’s Fair . 67 19 Dark Matter . 69 20 Guns and Roses . 71 21 Rampage . 73 22 Backscatter . 75 23 Undercurrents . 77 24 Hot Shot . 81 Numb3rs Episode Guide Season 3 83 1 Spree ............................................. -

Aerial Signatures of Forest Damage in Colorado and Adjoining States
Aerial Signatures of Forest Damage in Colorado and Adjoining States William M. Ciesla, S. Sky Stephens, Brian E. Howell and Justin C. Backsen Aerial Signatures of Forest Damage in Colorado and Adjoining States William M. Ciesla, S. Sky Stephens, Brian E. Howell and Justin C. Backsen April 2015 For additional copies of this publication, contact: Colorado State Forest Service Colorado State University 5060 Campus Delivery Fort Collins, CO 80523 970.491.6303 i Authors William M. Ciesla is owner of Forest Health Management International (FHMI), Fort Collins, Colo., and has been involved with aerial forest health surveys since 1959. He has conducted these surveys under contract to the Colorado State Forest Service since 2004. S. Sky Stephens was forest entomologist with the Colorado State Forest Service from 2009-2013, and is presently forest entomologist with the USDA Forest Service, Forest Health Protection, Rocky Mountain Region, Lakewood, Colo. She conducts aerial survey missions in the Region. Brian E. Howell is aerial survey program manager for the USDA Forest Service, Forest Health Protection in the Rocky Mountain Region, and has been conducting aerial survey missions in the Region since 2004. Justin C. Backsen is a forestry technician with the USDA Forest Service, Forest Health Protection in the Rocky Mountain Region, and conducts aerial survey missions throughout the Region. Aerial Signatures of Forest Damage in Colorado and Adjoining States ii Preface “Aerial Signatures of Forest Damage in Colorado and Adjoining States” is a revision and update of the USDA Forest Service, Forest Health Technology Enterprise Team (FHTET), publication FHTET-01-06 “Aerial Signatures of Forest Insect and Disease Damage in the Western United States” (Ciesla 2006). -

Scorched by Wajdi Mouawad Translated by Linda Gaboriau
Scorched by Wajdi Mouawad translated by Linda Gaboriau an NAC English Theatre / Tarragon Theatre (Toronto) English-language world premiere coproduction Study Guide THE NATIONAL ARTS CENTRE ENGLISH THEATRE PROGRAMMES FOR STUDENT AUDIENCES 2006-2007 SEASON Peter Hinton Artistic Director, English Theatre This Study Guide was written and researched by Jim McNabb for the National Arts Centre, English Theatre, February, 2007. It may be used solely for educational purposes. The National Arts Centre English Theatre values the feedback of teachers on the content and format of its Study Guides. We would appreciate your comments on past Study Guides, on this current one, or suggestions on ways to improve future Study Guides. Comments may be directed to Martina Kuska either by email at [email protected] or by fax at (613) 943-1401. Theatre5 Media Partner part of the NAC’s About This Guide This study guide is formatted in easy-to-copy single pages. They may be used separately or in any combination that works for your classes. The two colour pages found at the end of the Guide are intended for display in the classroom but may also be copied for distribution. Table of Contents page(s) About the Play ....................................................................................................1 œ 2 Setting; Plot Synopsis; Characters....................................................................1 Characters (continued); Style; Themes................................................................2 Some Things to Watch For in the production -

Fire and Animal Behavior
Proceedings: 9th Tall Timbers Fire Ecology Conference 1969 Komarek, E.v. 1969. Fire and animal behavior. Pages 160-207 in E.V. Komarek (conference chariman). Proceedings Tall Timbers Fire Ecology Conference: No.9. Tall Timbers Fire Ecology Conference. 9. Tall Timbers Research Station, Tallahassee, FL. Fire and Animal Behavior E. V. KOMAREK, SR. Tall Ti'mbers Researcb Station Tallabassee, Florida , THE REACTIONS and relationships of animals to fire, the charcoal black burn, the straw-colored and later the green vegetation certainly attracted the attention of primitive man and his ancestors. In "Fire-and the Ecology of iV'lan" I proposed that man himself is the product of a fire environment-"pre-'man prirrtates as well as early Ulan inhabited fire environrnents and had to be 'fire selected' ... to live in such environments." (Komarek, 1967). Therefore, man's ultimate survival, as had been true with other ani mals that live in fire environments, depends upon a proper under standing of fire and the relationships of animals and plants to this element. 1V10dern literature, particularly in the United States, has been so saturated with the reputed dangers of forest fires to animals that he has developed a deep seated fear of all fires in nature. In this nation, the average individual is only beginning to realize the proper place of fire and fire control in forest, grassland and field. Unfortunately, much knowledge on fire and animal behavior that was common in formation to the American Indian, pioneers, and early farm and ranch people has been lost though in some parts of the ·world a large body of this knowledge still exists.