Concerns Related to the Management of a Patient's Medication at Three
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

TRANDATE® (Labetalol Hydrochloride) Tablets
NDA 18716/S-026 Page 2 PRODUCT INFORMATION TRANDATE® (labetalol hydrochloride) Tablets DESCRIPTION: Trandate Tablets are adrenergic receptor blocking agents that have both selective alpha1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1 methyl-3-phenylpropyl)amino]ethyl]benzamide monohydrochloride, and it has the following structure: Labetalol HCl has the empirical formula C19H24N2O3•HCl and a molecular weight of 364.9. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R′ stereoisomer, makes up 25% of racemic labetalol. Labetalol HCl is a white or off-white crystalline powder, soluble in water. Trandate Tablets contain 100, 200, or 300 mg of labetalol HCl and are taken orally. The tablets also contain the inactive ingredients corn starch, FD&C Yellow No. 6 (100- and 300-mg tablets only), hydroxypropyl methylcellulose, lactose, magnesium stearate, pregelatinized corn starch, sodium benzoate (200-mg tablet only), talc (100-mg tablet only), and titanium dioxide. CLINICAL PHARMACOLOGY: Labetalol HCl combines both selective, competitive, alpha1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous (IV) administration, respectively. Beta2-agonist activity has been demonstrated in animals with minimal beta1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane stabilizing effect has been demonstrated. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -
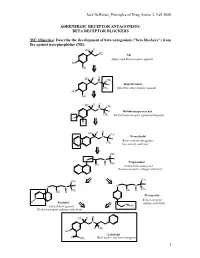
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -

Do Beta Blockers Cause Depression?
Medicine in Brief Do beta blockers cause depression? Andrew J. Muzyk, PharmD, and Jane P. Gagliardi, MD Principal Source: van Melle JP, Verbeek DEP, van den Berg ever, these reviews had major limitations, MP, et al. Beta-blockers and depression after myocardial such as inadequately defined methods for infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214. defining depression and lack of control for potential confounding factors. Mechanistically, peripheral effects of eyond their well-known role for treat- beta blockers on the heart and kidneys Bing cardiovascular disease, beta adren- lead to decreased chronotropy and inotro- Robert M. McCarron, DO ergic receptor antagonists—beta block- py as well as lower blood pressure. These Series Editor ers—are used for a variety of medical cardiovascular and hemodynamic changes conditions, including coronary artery dis- could cause fatigue, decreased energy, and ease, hypertension, migraines, and tremor. sexual dysfunction that may be interpreted Their usefulness makes them 1 of the most as symptoms of new-onset depression. commonly prescribed medication classes. Researchers found that beta-blocker Unfortunately, their increased use comes use was not associated with depression with increased reports of depression. Be- in a case-control study examining 4,302 ing able to sort fact from fiction will help New Jersey Medicaid records.3 Also, be- guide your care for patients taking beta cause most patients in this study received blockers who report new or worsening de- propranolol, the authors were unable to pressive symptoms. confirm a long-held belief that highly lipo- philic beta blockers (such as propranolol, Does research support a link? First reported in the 1960s, beta blocker- Practice Points induced depression was thought to re- • Although patients with cardiovascular sult from the drugs’ antagonistic effect on disease are at increased risk for developing norepinephrine at ß1 post-synaptic brain depression, there is no convincing receptors. -

References Disclosures
PND85 Prophylactic migraine medication use in primary care setting in Finland Wahlman Hanna1 , Purmonen Timo1 , Korolainen Minna A.1 , Forsström Jari2 1 Novartis Finland Oy, Espoo, Finland 2 Multirec Oy, Turku, Finland Backround Discussion and limitations References • Migraine is a disabling health condition. While it is the most • This study was based on primary health care prescriptions Linde et al. The cost of headache disorder in Europe: common among working-aged people, it presents significant and thus, excludes those patients who have only visited The Eurolight project. Eur J Neurol. 2012 19(5):703-11. indirect costs on society, especially when the attacks are specialized health care units or private health care providers. frequent (1). The results are descriptive and further studies is needed to evaluate potential differences in the patient subgroups. • It is important to define the current treatment when new Disclosures therapies are entering the market. • While these results provide an overview of prophylactic migraine medication use in primary health care, no • Aim of this study was to assess the use of prophylactic conclusion can be made on potential outcomes related • This research was initiated and funded by treatments and assess the number of prophylactic to these treatments. Novartis Finland Oy. treatment lines in a primary care setting in Finland. • Data extraction and data analysis were performed by Multirec Oy. Material and methods Conclusions: • Poster presented at: International Society for Pharmacoeconomics and Outcomes Research 21st Annual European Congress 10-14 November, 2018, • We conducted a retrospective register-based study on • Variety of different medications are used for migraine Barcelona, Spain. -

Hf Heart Failure
HEART FAILURE Performance Measure: Evidence- HF Based Beta Blocker at Discharge The ACC/AHA 2009 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult recommends using 1 of 3 Evidence-Based Beta Blockers proven to reduce mortality,( bisoprolol, carvedilol, and metoprolol succinate) for all stable patients with current or prior symptoms of HF and reduced LVEF, unless contraindicated (Level of Class 1 Level of Evidence: A). Q: What are the contraindications specific to Evidence-Based Beta Blockers? A: It is extremely unlikely that all three Evidence-Based Beta Blockers would not be tolerated in a patient, but a non-evidenced based beta blocker would be tolerated. This scenario is so rare that there is not a need to separate contraindications by beta blocker class (evidenced based and non-evidence based). In the extremely rare instance all three Evidence-Based Beta Blockers are contraindicated in the patient, you can select “Yes”, if the contraindication is documented by a physician, APN or PA and expressly references all three beta blocker medications e.g. “No - Bisoprolol, Coreg or Toprol XL - all not tolerated” or reference to the contraindication specifically states that as a class “evidenced based beta blockers” are contraindicated (e.g. “evidenced based beta blockers all tried previously, patient is intolerant to all”) . This should almost never occur. Q: A physician didn’t prescribe an Evidence-Based Beta Blocker due to the higher cost of those drugs. Is this a contraindication? A: Cost is NOT an acceptable reason for not prescribing an Evidence-Based Beta Blocker at hospital discharge. -

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Pharmacological Category Formulary Brief (if Notes / Similar NCF Medications Active? Medication Name (up-to-date) available) Miscellaneous Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized Beta2 Agonist Yes solution Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Gout (2016) Yes Inhibitor Alogliptin Antidiabetic Agent, Dipeptidyl DPP-IV Inhibitors Yes Peptidase 4 (DPP-4) Inhibitor (2019) Amitriptyline Antidepressant, Tricyclic Antidepressants in Nortriptyline Yes Pain Mgmt (2014) Amlodipine Antihypertensive; Calcium Calcium Channel Diltiazem Yes Channel Blocker, Dihydropyridine Blockers (2014) Anastrozole Antineoplastic Agent, Aromatase Yes Inhibitor Apixaban Anticoagulant; Direct Oral Direct Oral Warfarin Yes Anticoagulant (DOAC); Factor Xa Anticoagulants (2017) Inhibitor Aspirin Antiplatelet Agent; Nonsteroidal Yes Anti-Inflammatory Drug; Salicylate Atenolol Antihypertensive; Beta-Blocker, Beta Blockers (2014) Metoprolol; Propranolol Yes Beta1 Selective Atomoxetine Norepinephrine Reuptake Inhibitor, ADHD (2020) Dextroamphetamine / Amphetamine (immediate release); Yes Selective Dextroamphetamine / Amphetamine (long-acting); Methylphenidate (immediate release); Methylphenidate (long-acting) Atorvastatin Antilipemic Agent, HMG-CoA Dyslipidemia Guideline Pravastatin; Rosuvastatin; Simvastatin Yes Reductase Inhibitor -

Beta-Blockers for Cardiovascular Conditions
CARDIOVASCULAR SYSTEM PHARMACOLOGY MEDICINE INDICATIONS Beta-blockers for cardiovascular conditions: one size does not fit all patients Metoprolol succinate accounts for almost three-quarters of the beta-blockers dispensed in New Zealand. There is, however, little evidence to support the systematic use of metoprolol succinate over other medicines in this class. Prescribers are encouraged to use the pharmacological diversity of beta-blockers and the clinical characteristics of patients to individualise treatment and optimise care. KEY PRACTICE POINTS: Metoprolol succinate is the most frequently prescribed beta-blocker in New Zealand Beta-blockers are a diverse group of medicines and prescribers should consider their different properties, along In 2016, 325 000 people received a beta-blocker from a with the presence of co-morbidities, to individualise care for community pharmacy in New Zealand:1 patients with cardiovascular conditions 72% were prescribed metoprolol succinate; the seventh When a beta-blocker is initiated, a slow upwards titration most prescribed medicine in New Zealand of dose is recommended to minimise adverse effects. 7% were prescribed bisoprolol Beta-blockers should also be withdrawn slowly, ideally over several months, to prevent rebound symptoms such as 5% were prescribed atenolol resting tachycardia. 4% were prescribed carvedilol or propranolol From 6-12 months onwards post-myocardial infarction, consider withdrawing beta-blockers for patients without This pattern of prescribing is different to other countries, such