207533Orig1s000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -

Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients Research Questions: 1. How many schizophrenia patients are prescribed recommended first-line second-generation treatments for schizophrenia? 2. How many schizophrenia patients switch to an injectable antipsychotic after stabilization on an oral antipsychotic? 3. How many schizophrenia patients are prescribed 2 or more concomitant antipsychotics? 4. Are claims for long-acting injectable antipsychotics primarily billed as pharmacy or physician administered claims? 5. Does adherence to antipsychotic therapy differ between patients with claims for different routes of administration (oral vs. long-acting injectable)? Conclusions: In total, 4663 schizophrenia patients met inclusion criteria, and approximately 14% of patients (n=685) were identified as treatment naïve without claims for antipsychotics in the year before their first antipsychotic prescription. Approximately 45% of patients identified as treatment naïve had a history of remote antipsychotic use, but it is unclear if antipsychotics were historically prescribed for schizophrenia. Oral second-generation antipsychotics which are recommended as first-line treatment in the MHCAG schizophrenia algorithm were prescribed as initial treatment in 37% of treatment naive patients and 28% of all schizophrenia patients. Recommended agents include risperidone, paliperidone, and aripiprazole. Utilization of parenteral antipsychotics was limited in patients with schizophrenia. Overall only 8% of patients switched from an oral to an injectable therapy within 6 months of their first claim. Approximately, 60% of all schizophrenia patients (n=2512) had claims for a single antipsychotic for at least 12 continuous weeks and may be eligible to transition to a long-acting injectable antipsychotic. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

TRANDATE® (Labetalol Hydrochloride) Tablets
NDA 18716/S-026 Page 2 PRODUCT INFORMATION TRANDATE® (labetalol hydrochloride) Tablets DESCRIPTION: Trandate Tablets are adrenergic receptor blocking agents that have both selective alpha1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1 methyl-3-phenylpropyl)amino]ethyl]benzamide monohydrochloride, and it has the following structure: Labetalol HCl has the empirical formula C19H24N2O3•HCl and a molecular weight of 364.9. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R′ stereoisomer, makes up 25% of racemic labetalol. Labetalol HCl is a white or off-white crystalline powder, soluble in water. Trandate Tablets contain 100, 200, or 300 mg of labetalol HCl and are taken orally. The tablets also contain the inactive ingredients corn starch, FD&C Yellow No. 6 (100- and 300-mg tablets only), hydroxypropyl methylcellulose, lactose, magnesium stearate, pregelatinized corn starch, sodium benzoate (200-mg tablet only), talc (100-mg tablet only), and titanium dioxide. CLINICAL PHARMACOLOGY: Labetalol HCl combines both selective, competitive, alpha1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous (IV) administration, respectively. Beta2-agonist activity has been demonstrated in animals with minimal beta1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane stabilizing effect has been demonstrated. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -

Second-Generation Long-Acting Injectable Antipsychotics: a PRACTICAL GUIDE Understanding Each of These Medications’ Unique Properties Can Optimize Patient Care
Second-generation long-acting injectable antipsychotics: A PRACTICAL GUIDE Understanding each of these medications’ unique properties can optimize patient care Brittany L. Parmentier, PharmD, MPH, here are currently 7 FDA-approved second-generation long-acting BCPS, BCPP 1-7 Clinical Assistant Professor injectable antipsychotics (LAIAs). These LAIAs provide a Department of Pharmacy Practice Tunique dosage form that allows patients to receive an antipsy- The University of Texas at Tyler Fisch College of Pharmacy chotic without taking oral medications every day, or multiple times per Tyler, Texas day. This may be an appealing option for patients and clinicians, but Disclosure The author reports no financial relationships with any because there are several types of LAIAs available, it may be difficult to companies whose products are mentioned in this article, or with determine which LAIA characteristics are best for a given patient. manufacturers of competing products. Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved: • aripiprazole LAI • aripiprazole lauroxil LAI • olanzapine pamoate LAI • paliperidone palmitate monthly injection • paliperidone palmitate 3-month LAI • risperidone LAI for subcutaneous (SQ) injection. When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications. A major potential benefit: Increased adherence One potential benefit of all LAIAs is increased medication adherence com- pared with oral antipsychotics. One meta-analysis of 21 randomized con- trolled trials (RCTs) that compared LAIAs with oral antipsychotics and Current Psychiatry GEORGE MATTEI/SCIENCE SOURCE GEORGE MATTEI/SCIENCE Vol. -

Should Inverse Agonists Be Defined by Pharmacological Mechanism Or
CNS Spectrums,(2019), 24 419 – 425. © Cambridge University Press 2018. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited. doi:10.1017/S1092852918000986 ORIGINAL RESEARCH Switching stable patients with schizophrenia from their oral antipsychotics to aripiprazole lauroxil: a post hoc safety analysis of the initial 12-week crossover period Peter J. Weiden,1* Yangchun Du,2 Chih-Chin Liu,2 and Arielle D. Stanford3 1 Medical Affairs, Alkermes, Inc., Waltham, Massachusetts, USA 2 Clinical Development, Alkermes, Inc., Waltham, Massachusetts, USA 3 Clinical Science, Alkermes, Inc., Waltham, Massachusetts, USA Objective. Switching antipsychotic medications is common in patients with schizophrenia who are experiencing persistent symptoms or tolerability issues associated with their current drug regimen. This analysis assessed the safety of switching from an oral antipsychotic to the long-acting injectable antipsychotic aripiprazole lauroxil (AL). Methods. This was a post hoc analysis of outpatients with schizophrenia who were prescribed an oral antipsychotic and who enrolled in an international, open-label, long-term (52-week) safety study of AL. The analysis focused on the first 3 injections of AL 882 mg over 12 weeks, divided into the immediate 4-week crossover period between the first and second AL injections (initiation phase) and the subsequent 8 weeks (stabilization phase). Patients were grouped by preswitch oral antipsychotic medication, and safety and clinical symptoms were assessed. Results. In total, 190 patients had switched from one of the following oral antipsychotic medications: aripiprazole, conventional antipsychotics, risperidone/paliperidone, olanzapine, or quetiapine. -
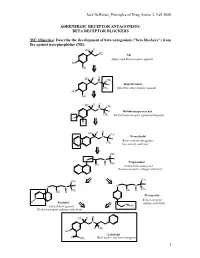
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Aristada Initio (Aripiprazole Lauroxil), in Combination with Oral Aripiprazole for the Initiation of Aristada When Used for the Treatment of Schizophrenia in Adults
Aristada Initio™ (aripiprazole lauroxil) – New drug approval • On July 2, 2018, Alkermes announced the FDA approval of Aristada Initio (aripiprazole lauroxil), in combination with oral aripiprazole for the initiation of Aristada when used for the treatment of schizophrenia in adults. — The approval of Aristada Initio provides physicians with an alternative regimen to initiate patients onto any dose of Aristada injection on day 1. — Previously, the first Aristada injection was recommended in conjunction with oral aripiprazole for 21 consecutive days. • Oral aripiprazole is generically available as tablets, disintegrating tablets, and solution. Aripiprazole is also available as Abilify Maintena®, a brand extended-release injectable suspension. — The oral formulations are approved for the treatment of schizophrenia and other indications as outlined in the individual drug labels. — Abilify Maintena is indicated for the treatment of schizophrenia in adults and as maintenance monotherapy treatment of bipolar I disorder in adults. • Aristada Initio leverages Alkermes’ proprietary NanoCrystal® technology, which provides an extended-release formulation using a smaller particle size compared to Aristada, thereby enabling faster dissolution and more rapid achievement of relevant drug levels. • The effectiveness of Aristada Initio, in combination with oral aripiprazole and for the initiation of Aristada injection, was established by adequate and well-controlled studies of oral aripiprazole and Aristada injection in adult patients with schizophrenia. -

Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia
brain sciences Review Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia Samskruthi Madireddy 1,* and Sahithi Madireddy 2 1 Independent Researcher, 1353 Tanaka Drive, San Jose, CA 95131, USA 2 Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, MA 02139, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-408-9214162 Received: 4 September 2020; Accepted: 15 October 2020; Published: 16 October 2020 Abstract: The biochemical integrity of the brain is paramount to the function of the central nervous system, and oxidative stress is a key contributor to cerebral biochemical impairment. Oxidative stress, which occurs when an imbalance arises between the production of reactive oxygen species (ROS) and the efficacy of the antioxidant defense mechanism, is believed to play a role in the pathophysiology of various brain disorders. One such disorder, schizophrenia, not only causes lifelong disability but also induces severe emotional distress; however, because of its onset in early adolescence or adulthood and its progressive development, consuming natural antioxidant products may help regulate the pathogenesis of schizophrenia. Therefore, elucidating the functions of ROS and dietary antioxidants in the pathogenesis of schizophrenia could help formulate improved therapeutic strategies for its prevention and treatment. This review focuses specifically on the roles of ROS and oxidative damage in the pathophysiology of schizophrenia, as well as the effects of nutrition, antipsychotic use, cognitive therapies, and quality of life on patients with schizophrenia. By improving our understanding of the effects of various nutrients on schizophrenia, it may become possible to develop nutritional strategies and supplements to treat the disorder, alleviate its symptoms, and facilitate long-term recovery.