TRANDATE® (Labetalol Hydrochloride) Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

Pain Management Opioid Safety a Quick Reference Guide (2014)
Pain Management Opioid Safety A Quick Reference Guide (2014) VA Academic Detailing Service Real Provider Resources Real Patient Results Your Partner in Enhancing Veteran Health Outcomes VA Academic Detailing Service Email Group: [email protected] VA Academic Detailing Service SharePoint Site: https://vaww.portal2.va.gov/sites/ad Opioids: A Practical Guide for Clinicians Example Risk Assessment Tool: Opioid Risk Tool (ORT)1 Item Score if Female Item Score if Male Alcohol 1 3 1. Family history of substance abuse Illegal drugs 2 3 Prescription drugs 4 4 Alcohol 3 3 2. Personal history of substance abuse Illegal drugs 4 4 Prescription drugs 5 5 3. Age (mark box if 16–45) 1 1 4. History of preadolescent sexual abuse 3 0 Attention deficit disorder obsessive compulsive disorder 2 2 5. Psychological disease Bipolar Schizophrenia Depression 1 1 Total Risk Category: 0–3 Low Risk of aberrant behaviors; 4–7 Moderate Risk of aberrant behaviors; ≥8 High Risk of aberrant behaviors Assess risk of aberrant behaviors before initiating opioid medications; the ORT or other rating tools can assist with this process but can overestimate risk thus should not be used as only reason to decline opioid prescription. 1 Opioids Risk Classification10-11 Risk Condition/Situation • Diagnosis with concordant physical exam, medical imaging, laboratory findings • High levels of pain acceptance and active coping strategies Low • Well motivated patient willing to participate in multimodal treatment plan (no moderate to high risk • Attempting to function -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

New Zealand Data Sheet 1. Product Name
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Norvir 100 mg tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 100 mg ritonavir. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM White film-coated oval tablets debossed with the corporate Abbott "A" logo and the Abbott-Code “NK”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications NORVIR is indicated for use in combination with appropriate antiretroviral agents or as monotherapy if combination therapy is inappropriate, for the treatment of HIV-1 infection in adults and children aged 12 years and older. For persons with advanced HIV disease, the indication for ritonavir is based on the results for one study that showed a reduction in both mortality and AIDS defining clinical events for patients who received ritonavir. Median duration of follow-up in this study was 6 months. The clinical benefit from ritonavir for longer periods of treatment is unknown. For persons with less advanced disease, the indication is based on changes in surrogate markers in controlled trials of up to 16 weeks duration (see section 5.1 Pharmacodynamic properties). 4.2 Dose and method of administration General Dosing Guidelines Prescribers should consult the full product information and clinical study information of protease inhibitors if they are co-administered with a reduced dose of ritonavir. The recommended dose of NORVIR is 600 mg (six tablets) twice daily by mouth, and should be given with food. NORVIR tablets should be swallowed whole and not chewed, broken or crushed. NORVIR DS 29 April 2020 Page 1 of 37 Version 35 Paediatric population Ritonavir has not been studied in patients below the age of 12 years; hence the safety and efficacy of ritonavir in children below the age of 12 have not been established. -

Adrenoceptors Regulating Cholinergic Activity in the Guinea-Pig Ileum 1978) G.M
- + ! ,' Br. J. Pharmac. (1978), 64, 293-300. F'(O t.,," e reab- ,ellular PHARMACOLOGICAL CHARACTERIZATION OF THE PRESYNAPTIC _-ADRENOCEPTORS REGULATING CHOLINERGIC ACTIVITY IN THE GUINEA-PIG ILEUM 1978) G.M. Departmentof Pharmacology,Allen and HzmburysResearchLimited, Ware, Hertfordshire,SG12 ODJ I The presynaptic ct-adrenoceptors located on the terminals of the cholinergic nerves of the guinea- pig myenteric plexus have been characterized according to their sensitivities to at-adrenoceptor agonists and antagonists. 2 Electrical stimulation of the cholinergic nerves supplying the longitudinal muscle of the guinea-pig ! ileum caused a twitch response. Clonidine caused a concentration-dependent inhibition of the twitch i response; the maximum inhibition obtained was 80 to 95_o of the twitch response. Oxymetazoline and xylazine were qualitatively similar to clonidine but were about 5 times less potent. Phenylephrine and methoxamine also inhibited the twitch response but were at least 10,000 times less potent than clonidine. 3 The twitch-inhibitory effects of clonidine, oxymetazoline and xylazine, but not those of phenyl- ephrine or methoxamine, were reversed by piperoxan (0.3 to 1.0 lag/ml). 4 Lysergic acid diethylamide (LSD) inhibited the twitch response, but also increased the basal tone of the ileum. Mepyramine prevented the increase in tone but did not affect the inhibitory action of LSD. Piperoxan or phentolamine only partially antagonized the inhibitory effect of LSD. 5 Phentolamine, yohimbine, piperoxan and tolazoline were potent, competitive antagonists of the inhibitory effect of clonidine with pA2 values of 8.51, 7.78, 7.64 and 6.57 respectively. 6 Thymoxamine was a weak antagonist of clonidine; it also antagonized the twitch-inhibitory effect of morphine. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -

Urine Drug Testing – Ordering and Interpretation The
URINE DRUG TESTING – ORDERING AND INTERPRETATION Created by: Dr. Daniel Berland, M.D. THE TESTS Enzyme linked immunoassay (EIA) kits • Screening test for illicit substances amphetamine/methamphetamine, (marijuana, PCP, cocaine, “opiates” (morphine/codeine) • Inexpensive, fast, point of care or lab • Detects class of substance, not specific medication • Will be negative for hydrocodone, hydromorphone, oxycodone, methadone, buprenorphine, benzodiazepines (particularly clonazepam) unless specific test kit for those meds is in use. Ask your lab! • High false positive rates caused by numerous prescribed or OTC meds Gas chromatography/Mass Spectroscopy (GCMS) • More expensive, labor intensive • Confirming test identifies specific meds and their metabolites. Use to confirm patient is taking prescribed meds and not taking non-prescribed meds • High sensitivity, but you must tell the lab what you seek (patient is taking) • False positives still occur ** Human Urine: T ~98 deg; > 90 deg for 15 min. pH 4.5-8; SG 1.002-1.03. Ur Cr > 20 mg/dL ** WHAT TO ORDER • Test for illicit drug use: EIA • Test to confirm taking prescribed meds: GCMS (EIA is OK if your lab runs the test for each med – they usually do not – ask!) • Test to check for use of non-prescribed medication: GCMS POSSIBLE OUTCOMES OF TESTING • Presence of illicit substance: Use by patient; false result related to prescribed or OTC med exposure • Presence of non-prescribed medication: Illicit use by patient; false positive testing – cross-reaction or possible known metabolite (e.g., morphine -
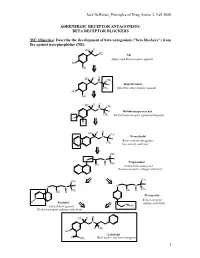
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Management of Postnatal Hypertension - Top Tips
Management of postnatal hypertension - top tips FOLLOW UP PLAN Follow up Hypertensive disorder Chronic Gestational Pre - Severe pre- hypertension hypertension eclampsia eclampsia/ Mild/ HELLP/ Moderate Eclampsia Initial follow up Community midwife and general practitioner BP frequency At least once 3- At least once Every 1-2 days for 2 wks 5 days post 3-5 days post as above birth then as birth clinically Then as indicated clinically indicated Acceptable BP Aim BP should be <150/100 mmHg. BP <140/90 If not on antihypertensives commence if BP≥ 150/100 Suitable Labetalol, nifedipine, enalapril, atenolol, antihypertensives AVOID DIURETICS STOP METHYLDOPA WITHIN 2 DAYS OF DELIVERY When to reduce/ NA If BP<130/80 If BP<130/80 reduce stop If not seen reduce antihypertensive before needs If BP < 140/ If BP <140/ 90 consider GP review at 90 consider reducing 2wks post birth reducing Convert back to If not seen prepregnancy before needs antihypertensive GP review at as indicated 2wks post birth 6 weeks GP/ pre GP GP Hospital cons follow up pregnancy care clinic team POSTNATAL REVIEW AT POSTNATAL REVIEW 6-8 weeks after birth Offer medical review Offer specialist assessment if antihypertensives still needed Rpt Plt, transaminases, creatinine if indicated Carry out urine dipstick test If proteinuria still ≥ 1+ offer further review at 3 months to assess kidney function – consider offering renal referral. Give advice re: recurrence risks – see table below: Advise women to maintain BMI 18.5-24.9 kg/m2 H.Yeeles/S. Rutter July 14 Management of postnatal -

(LSD) Test Dip Card (Urine) • Specimen Collection Container % Agreement 98.8% 99
frozen and stored below -20°C. Frozen specimens should be thawed and mixed before testing. GC/MS. The following results were tabulated: Method GC/MS MATERIALS Total Results Results Positive Negative Materials Provided LSD Rapid Positive 79 1 80 LSD • Test device • Desiccants • Package insert • Urine cups Test Dip card Negative 1 99 100 Materials Required But Not Provided Total Results 80 100 180 One Step Lysergic acid diethylamide (LSD) Test Dip card (Urine) • Specimen collection container % Agreement 98.8% 99. % 98.9% • Timer Package Insert DIRECTIONS FOR USE Analytical Sensitivity This Instruction Sheet is for testing of Lysergic acid diethylamide. Allow the test device, and urine specimen to come to room temperature [15-30°C (59-86°F)] prior to testing. A drug-free urine pool was spiked with LSD at the following concentrations: 0 ng/mL, -50%cutoff, -25%cutoff, cutoff, A rapid, one step test for the qualitative detection of Lysergic acid diethylamide and its metabolites in human urine. 1) Remove the test device from the foil pouch. +25%cutoff and +50%cutoff. The result demonstrates >99% accuracy at 50% above and 50% below the cut-off For forensic use only. 2) Remove the cap from the test device. Label the device with patient or control identifications. concentration. The data are summarized below: INTENDED USE 3) Immerse the absorbent tip into the urine sample for 10-15 seconds. Urine sample should not touch the plastic Lysergic acid diethylamide (LSD) Percent of Visual Result The One Step Lysergic acid diethylamide (LSD) Test Dip card (Urine) is a lateral flow chromatographic device. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational