A Report of Two Children with Gorham-Stout Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Benign Bone Tumors of the Foot and Ankle
CHAPTER 20 BENIGN BONE TUMORS OF THE FOOT AND ANKLE, Robert R. Miller, D.P.M. Stephen V. Corey, D.P.M. Benign bone tumors of the foot and ankle typically Table 1 displays the percentage of each lesion present both a diagnostic and therapeutic challenge found in the leg and foot. The lesions represent a to podiatric surgeons. These lesions have a percentage of local lesions compared to the total relatively low incidence of occuffence in the foot number of lesions reported for the studies. It does and ankle when compared to other regions of the seem apparent that the overall incidence of foot body, and the behavior of these lesions may mimic and ankle involvement is relatively low, but some malignant tumors. Not only is it impofiant to tumors do occur with a somewhat frequent rate. recognize a specific lesion to insure proper treat- Primarily, enchondroma, osteochondroma, osteoid ment, but the ability to differentiate a benign from osteoma, simple (unicameral) bone cysts, and malignant process is of utmost importance. aneurysmal bone cysts are somewhat common in It is difficult to determine the true incidence of the foot and ankle. benign bone tumors of the foot and ankle. Most large studies do not distinguish individual tarsal RADIOGRAPHIC CHARACTERISTICS OF bones, nor is there a distinction made befween BENIGN BONE TUMORS proximal and distal aspects of the tibia and fibula. Dahlin's Bone Tumors has reported findings of the Several radiographic parameters have been Mayo Clinic up until 7993.' total 2334 Of a of described to differentiate between benign and benign bone tumors affecting the whole body, malignant bone tumors. -

Tuberculosis – the Masquerader of Bone Lesions in Children MN Rasool FCS(Orth) Department of Orthopaedics, University of Kwazulu-Natal
SAOJ Autumn 2009.qxd 2/27/09 11:11 AM Page 21 CLINICAL ARTICLE SA ORTHOPAEDIC JOURNAL Autumn 2009 / Page 21 C LINICAL A RTICLE Tuberculosis – the masquerader of bone lesions in children MN Rasool FCS(Orth) Department of Orthopaedics, University of KwaZulu-Natal Reprint requests: Dr MN Rasool Department of Orthopaedics University of KwaZulu-Natal Private Bag 7 Congella 4001 Tel: (031) 260 4297 Fax: (031) 260 4518 Email: [email protected] Abstract Fifty-three children with histologically confirmed tuberculous osteomyelitis were treated between 1989 and 2007. The age ranged from 1–12 years. There were 65 osseous lesions (excluding spinal and synovial). Seven had mul- tifocal bone involvement. Four basic types of lesions were seen: cystic (n=46), infiltrative (n=7), focal erosions (n=6) and spina ventosa (n=7). The majority of lesions were in the metaphyses (n=36); the remainder were in the diaphysis, epiphysis, short tubular bones, flat bones and small round bones. Bone lesions resembled chronic infections, simple and aneurysmal bone cysts, cartilaginous tumours, osteoid osteoma, haematological bone lesions and certain osteochondroses seen during the same period of study. Histological confirmation is man- datory to confirm the diagnosis of tuberculosis as several bone lesions can mimic tuberculous osteomyelitis. Introduction The variable radiological appearance of isolated bone Tuberculous osteomyelitis is less common than skeletal lesions in children can resemble various bone lesions tuberculosis involving the spine and joints. The destruc- including subacute and chronic osteomyelitis, simple and tive bone lesions of tuberculosis, the disseminated and the aneurysmal bone cysts, cartilaginous tumours, osteoid multifocal forms, are less common now than they were 50 osteoma, granulomatous lesions, haematological disease, 6,7,12 years ago.1-7 However, in recent series, solitary involve- and certain malignant tumours. -

Management of Unicameral Bone Cyst of Proximal Femur: Experience of 14 Cases and Review of Literature
202 KUWAIT MEDICAL JOURNAL September 2008 Original Article Management of Unicameral Bone Cyst of Proximal Femur: Experience of 14 Cases and Review of Literature Magdy M Abdel-Mota’al, Abdul Salam Othman Mohamad, Kenneth Chukwuka Katchy, Amarnath A Mallur, Fawzy Hamido Ahmad, Barakat El-Alfy Kuwait Medical Journal 2008, 40 (3): 202-210 ABSTRACT Objective: To assess the results of surgical treatment Main Outcome Measures: Patients were followed up of unicameral bone cyst (UBC) involving the proximal post-operatively for an average period of 42 months (range femur = 9–120 months). They were observed for recurrence, Design: Retrospective study of 14 cases of UBC of complications and fracture healing. proximal femur Results: Recurrence was observed in one case while other Setting: Al-Razi Orthopedic Hospital, Kuwait cases showed healing of the cyst with consolidation and Subjects and Methods: Fourteen cases of UBC seen and varying degrees of remodeling in one years time. A case treated at Al-Razi hospital were included in the study. developed mal-union and growth arrest with subsequent Their presentation and the method of treatment were shortening. Avascular necrosis and coxa vara was recorded. detected in another case. All the fractures healed in the Intervention: Thirteen cases were treated surgically using usual expected time according to age. intra-lesional excision (ILE). The cavity was filled with Conclusion: UBC of the proximal femur exhibits unique autogenous bone graft in three cases, hydroxyapatite characters and complications. Hydroxyapatite matrix matrix (HA) in eight cases, and combined autogenous is a useful and effective bone substitute. Post-excision graft and hydroxyapatite matrix in two cases. -

Massive Axial and Appendicular Skeletal Deformities in Connection with Gorham-Stout Syndrome
Case Report Massive Axial and Appendicular Skeletal Deformities in Connection with Gorham-Stout Syndrome Ali Al Kaissi 1,2,*, Sami Bouchoucha 3, Mohammad Shboul 4, Vladimir Kenis 5, Franz Grill 2, Rudolf Ganger 2 and Susanne Gerit Kircher 6 1 Ludwig Boltzmann Institute of Osteology, at the Hanusch Hospital of WGKK and, AUVA Trauma Centre Meidling, First Medical Department, Hanusch Hospital, 1090 Vienna, Austria 2 Orthopaedic Hospital of Speising, Paediatric department, 1090 Vienna, Austria; [email protected] (F.G.); [email protected] (R.G.) 3 Paediatric Orthopedic Surgery—Children Hospital, Tunis 1029, Tunis-Tunisia; [email protected] 4 Department of Medical Laboratory Sciences, Jordan University of Science and Technology, Irbid 22110, Jordan; [email protected] 5 Department of Foot and Ankle Surgery, Neuroorthopaedics and Systemic Disorders, Pediatric Orthopedic Institute n.a. H. Turner, Parkovaya str., 64-68, Pushkin, Saint Petersburg, Russia; [email protected] 6 Department of Medical Chemistry, Medical University of Vienna, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel. (Fax): +43-180-182-1260 Received: 26 March 2019; Accepted: 5 May 2019; Published: 7 May 2019 Abstract: Background: Etiological understanding is the corner stone in the management of skeletal deformities. Methods: Multi-centre study of patients with deformities in connection with diverse etiological backgrounds. We aimed to study four patients (one boy and three girls) with variable axial and appendicular deformities in connection with a vanishing bone disorder. Results: Axial deformities such as scoliosis, kyphoscoliosis, compressed fused vertebrae, appendicular fractures, dislocations, and vicious disorganization deformities of the joints were in connection with the vanishing bone disorder, namely Gorham-Stout syndrome. -
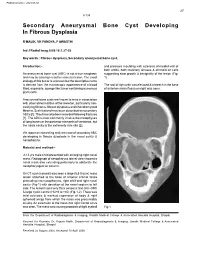
Secondary Aneurysmal Bone Cyst Developing in Fibrous Dysplasia
Published online: 2021-08-02 27 9-135 Secondary Aneurysmal Bone Cyst Developing In Fibrous Dysplasia R MALIK, VK PANDYA, P AWASTHI Ind J Radiol Imag 2006 16:1:27-28 Key words : Fibrous dysplasia, Secondary aneurysmal bone cyst. Introduction:- and pressure moulding with sclerosis of medial wall of both orbits, both maxillary sinuses & ethmoid air cells An aneurysmal bone cyst (ABC) is not a true neoplasm suggesting slow growth & benignitity of the lesion (Fig and may be a benign reactive vascular lesion. The exact 1). etiology of this tumor is unknown but the descriptive name is derived from the microscopic appearance of a blood The roof of right orbit was elevated & a breech in the base filled, expansile, sponge like tumor containing numerous of anterior cranial fossa on right was seen. giant cells. Aneurysmal bone cysts are known to arise in association with other abnormalities of the skeleton, particularly non ossifying fibroma, fibrous dysplasia and chondromyxoid fibroma. Such lesions have been described as secondary ABCs [1]. They have also been recorded following fractures [1]. The ABCs most commonly involve the metaphyses of long bones or the posterior elements of vertebrae, but the nasal cavity is the extremely rare site [2]. We report an interesting and rare case of secondary ABC developing in fibrous dysplasia in the nasal cavity & nasopharynx. Material and method:- A 13 yrs male child presented with enlarging right nasal mass. Radiograph of nasopharynx lateral view showed a nasal mass also extending posteriorly to obliterate the nasopharyngeal air column. On CT scan (coronal) was seen a large (5.5 X 6cm) mass lesion attached to the base of anterior cranial fossa protruding into nasopharynx, right orbit and right nasal cavity (Fig-1) with deviation of the nasal septum to left side. -

Pathologic Femoral Neck Fractures in Children
An Original Study Pathologic Femoral Neck Fractures in Children M. Wade Shrader, MD, Joseph H. Schwab, MD, William J. Shaughnessy, MD, and David J. Jacofsky, MD more common in adults and usually are caused by meta- ABSTRACT static disease. These fractures are typically treated with Pathologic fractures in children occur in a variety of internal fixation, intramedullary fixation, or arthroplasty.1 malignant and benign pathologic processes. Pediatric Fractures in the femoral neck of children are extremely pathologic femoral neck fractures are particularly rare and require special treatment considerations because rare. Until now, all reported cases have been isolated of growth issues and the possibility of avascular necrosis cases, small series, or cases reported in series of (AVN).2 adult pathologic hip fractures. The present article is the first report of a relatively large series of patho- The literature includes very little on pathologic femoral logic femoral neck fractures in a pediatric population. neck fractures in children. Fractures in FD have received We identified pathologic femoral neck fractures, the most attention. In their series on femoral neck insuf- including 2 basicervical fractures, in 15 children (9 ficiency fractures in FD, Enneking and Gearen3 indicated boys, 6 girls) ranging in age from 18 months to 15 years that 6 of the 15 patients were children. Funk and Wells4 (mean age, 9 years) and treated between 1960 and reported on 4 pediatric patients with FD, 2 of whom had 2000. The pathologic diagnoses were fibrous dysplasia pathologic femoral neck fractures. In addition, several (5 children), unicameral bone cyst (2), Ewing’s sarcoma other authors have documented single cases of pathologic (2), osteomyelitis (2), leukemia (1), rhabdomyosarcoma hip fracture in patients with simple bone cysts, aneurysmal (1), osteogenesis imperfecta (1), and osteopetrosis (1). -

Vanishing Bone Disease
Journal of Regenerative Biology and Medicine ISSN: 2582-385X Tamilthangam P, et al., 2021- J Regen Bio Med Review Article Vanishing Bone Disease: The unseen 1Senior lecturer, Department of Oral Seen Pathology and Microbiology, 1 2 Periyasamy Tamilthangam, * Jeyaraman Swathiraman Vivekanandha Dental College for Women, Thangavelu kavin3, Aravind RJ4, Ramesh Narendar5, India 6 7 S.P.Indra kumar and Meyyappan Suruthi keerthana 2Senior lecturer, Department of Oral Pathology and Microbiology, Vivekanandha Dental College for Women, Abstract India Vanishing bone disease (Gorham disease) is an uncommon bone pathology that is characterized by a continuous replacement of bone 3Professor and Head, Department of oral with the abundance of endothelial cells. It is usually nonfamilial, but and maxillofacial surgery, Vivekanandha Dental College for Women, India maybe familial and occurs periodically in children and young adults. The lesion is usually nonexpansile and monocentric, rarely polyostotic, 4Professor, Department of oral and but locally aggressive. Though asymptomatic cases have been maxillofacial surgery, Vivekanandha Dental College for Women, India reported, clinical symptoms include pain, functional disability and swelling of the involved area. Pathologic fracture of the involved bone 5Reader, Department of oral and has also been noted in some patients. Surgical treatment, as well as maxillofacial surgery, Vivekanandha radiotherapy, forms the main basis of treatment modality. Dental College for Women, India 6Senior lecturer, Department of oral and Keywords: Gorham disease; Pathology; Surgical treatment; maxillofacial surgery, Vivekanandha Radiotherapy; Endothelial cells. Dental College for Women, India 7Intern, Vivekanandha Dental College for Introduction Women, India Vanishing bone disease (Gorham disease) is an uncommon bone pathology that is characterized by a continuous *Corresponding Author: Tamilthangam P, et al, 1Senior lecturer, Department of replacement of bone with the abundance of endothelial cells. -

Management Strategy for Unicameral Bone Cyst
Management of unicameral bone cyst MANAGEMENT STRATEGY FOR UNICAMERAL BONE CYST Chin-Yi Chuo, Yin-Chih Fu, Song-Hsiung Chien, Gau-Tyan Lin, and Gwo-Jaw Wang Department of Orthopedic Surgery, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. The management of a unicameral bone cyst varies from percutaneous needle biopsy, aspiration, and local injection of steroid, autogenous bone marrow, or demineralized bone matrix to the more invasive surgical procedures of conventional curettage and grafting (with autogenous or allogenous bone) or subtotal resection with bone grafting. The best treatment for a unicameral bone cyst is yet to be identified. Better understanding of the pathology will change the concept of management. The aim of treatment is to prevent pathologic fracture, to promote cyst healing, and to avoid cyst recurrence and re-fracture. We retrospectively reviewed 17 cases of unicameral bone cysts (12 in the humerus, 3 in the femur, 2 in the fibula) managed by conservative observation, curettage and bone grafting with open reduction and internal fixation, or continuous decompression and drainage with a cannulated screw. We suggest percutaneous cannulated screw insertion to promote cyst healing and prevent pathologic fracture. We devised a protocol for the management of unicameral bone cysts. Key Words: unicameral bone cyst, UBC, simple bone cyst, SBC, cannulated screw (Kaohsiung J Med Sci 2003;19:289–95) The unicameral bone cyst (UBC) or simple bone cyst The treatment of UBC includes: conservative (SBC) is encountered in the pediatric age group, with management to allow spontaneous healing during the majority of cases occurring in the first two decades puberty; curettage and grafting with autogenous or of life — with most around the age of 12 years [1,2]. -

Subchondral Bone Cysts Seen in Conjunction with Osteoarthritis Or Other Joint Damage, from Intra- Osseous Ganglia Or Synovial Cysts of Bone
CHAPTER 44 SUBCI_IONDRAL BONE CYSTS Stepben J. Miller, D.P.M, Cysts found in the sub-articular bone adjacent to macroscopic and microscopic characteristics. The joints afflicted by osteoarthritis or rheumatoid cysts are usually solitary, but may be multilocular. arthritis were identified and described by Plewes in They commonly develop in the epiphyseal or meta- 1940, Coliins in 7949, and Landells in 1953 (Fig. 1). physeal areas of bone adjacent to joints. Generally, they have a whitish or bluish lining consisting of parallel bundles of collagen. The fibrocyes lying along the inner wall of the cavity sometimes form an incomplete lining of flattened, synovial-like cells. This makes for a smooth wall within which the cyst contains a thick gelatinous mucoid fluid. Still, there are those who distinguish the subchondral bone cysts seen in conjunction with osteoarthritis or other joint damage, from intra- osseous ganglia or synovial cysts of bone. The former are more likely to communicate with the joint through the articular surface in up to 400/o of the cases, and therefore may be filled with synovial fluid; whereas the latter communicate with the joint cases the Figure 1. Sub-articular bone cyst in the metatarsal head of patient with in less than 3o/o of the documented and rheumatoid arthritis. fluid is more commonly a viscous mucoid material. In fact, the Armed Forces Institute of Pathology, as However, related subchondral bone cysts with a late as 1977, separated as distinct entities the sub- synovial-like lining were subsequently described by chondral bone cysts and the synovial cyst of bone. -

Chronic Osteomyelitis : with Special Reference to Treatment
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1942 Chronic osteomyelitis : with special reference to treatment Richard R. Altman University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Altman, Richard R., "Chronic osteomyelitis : with special reference to treatment" (1942). MD Theses. 901. https://digitalcommons.unmc.edu/mdtheses/901 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. by Hichard F. Altnan Senior Thesis Presented to the College of Medicine University of IJebrA.ska Omaha, 1942 CONTl::NTS page I. INT:LWDUCTIOJJ •• . 1 II. ETIOLOGY ••••• . .. .. 3 Cormnon Causes of OsteoJ'llYelitis •• .. .. .. 3 Unusual Causative Agents in Osteom.yelitis. • • • 6 Predisposing Causes •••••••••••••••••••••••••• 12 IncidP-nce ••• .. .. •• 14 III. PATHOLOGY •••••• . .. .. .. .. .. •• 18 Acute Bncl Chronic Osteomyelitis ••• .. .. • .18 Brodies' Abscess ••••••••••••••••••••••••• • • 22 Sclerosinfj Non-sup:pur11tive Osteomyelitis. • • 22 IV. SYl.1PT01.'IS JJJD DIAG1JOSIS ••• . .. .•• 24 Acute Osteomyelitis •• . .. .. .. .. • • 24 Chronic Osteomyelitis •• .. .. .. .. .. • .25 v. HISTORY OF TH CATMJi;HT •• ~ ••••• . .. .. .26 VI. TJ(]~fl Tl~{T~I,TT •••••••••••••••••• . .. TreRtment of Acute Osteonyelitis ••• . • .40 TreRtnent of Chronic Osteonyelitis. .. .43 Common Metho<l.s of TreAtMent •••• . .. • • • 45 Less ':!idely Used Hethods of TrP-Atment •••• 54 Factors For and A~ainst the VAribus Methods of TrentBent ••••••••• . .. .. .62 Results of TreAtment. -

Differential Diagnosis of Oral Enlargements in Children
Theme Section Differential diagnosisof oral enlargementsin children Catherine M. Flaitz, DDS, MS Gary C. Coleman,DDS, MS Abstract Oncea specific category has been selected, the char- The purposeof this article is to review soft tissue and acteristics of the lesion can be compared with other bony enlargements that typically occur in the oral and diseases that share commonclinical features and be- perioral region of children. In order to organize these le- havioral patterns. sions into a thoroughbut comprehensibleformat, the prin- Soft tissuelesions ciples of differential diagnosis must be used. All oral en- largementsare broadly classified as soft tissue or bony Papillary enlargementsof the soft tissues are a dis- abnormalities.Determination of the specific lesion category tinct group of lesions that are easy to recognize because is based primarily on a prominent feature that demon- of their commonclinical appearance. Most of these strates the nature of the lesion, followed by the secondary lesions represent a viral-induced epithelial prolifera- clinical features and any contributory patient information. tion resulting in pale, spongy-to-firm enlargementswith Classificationof exophyticsoft tissue entities includes:pap- a pebbly or papillary, rough surface texture. These illary surface enlargements, acute inflammatoryenlarge- slow-growinglesions are painless with a limited growth ments, reactive hyperplasias, benign submucosalcysts and potential. Broadly this group is divided into isolated neoplasms, and aggressive and malignant neoplasms. Bony or multiple lesions to assist in the diagnosis and appro- enlargementsof the maxilla and mandibleare divided into priate managementof the pediatric patient (Fig 2). The three categories: inflammatorylesions, benign cystic and behavior of these lesions is variable, ranging from spon- neoplastic lesions, and aggressive and malignantlesions. -

Laboratory Evaluation of Osteoporosis in Women
Laboratory Evaluation of Osteoporosis in Women Case: A 65 y/o woman steps off of a curb, falls and severe pain develops in her left hip. After a 911 call, EMS brings the woman to the local ED. What is the most likely underlying case of this clinical scenario? William E. Winter, MD University of Florida Departments of Pathology and Pediatrics 2-15-2015 Learning objectives: At the conclusion of this presentation, the laboratorian will be able to: 1. Describe the circumstances when the diagnosis of osteoporosis should be considered. 2. List the causes of pathological fractures. 3. Define osteoporosis. 4. Discuss the causes of osteoporosis. 5. Interpret the results of laboratory testing that is conducted during the evaluation of pt’s w/ possible osteoporosis. 6. Interpret bone marker testing results. 7. Understand the controversy that relates to the value of bone marker testing in the evaluation of osteoporosis and its therapy. Under what clinical circumstances is the diagnosis of osteoporosis considered in women? Women w/: (1) Loss in height, kyphosis, other acquired skeletal deformities. (2) Low BMD on DEXA scan. (3) Fractures. Fracture Pathologic Traumatic (“Routine” Force > bone strength) (“Exceptional” Force > bone strength) Stepping off a curb Falling off of a tall ladder ‐ > hip fracture ‐ > hip fracture BMD = bone mineral density What are the causes of pathologic fractures? Focal bone disease Neoplasms ‐ Malignant ‐ Benign Non‐neoplastic disease (e.g., bone cyst, osteomyelitis; dysostoses, osteonecrosis) Generalized bone disease Congenital disorders (e.g., dysplasias) Acquired disorders See: http://www.wheelessonline.com/ortho/pathologic_fracture What are examples of neoplasms that can cause pathologic fractures? Malignant ‐ Bone cancers ‐ Malignant fibrous histiocytoma (a.k.a.