Engineer's Guide to Corrosion-Causes, Protection and Control Eight (8) Continuing Education Hours Course #ME1112
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aero-Flex Corporation 3147 Jupiter Park Circle Suite 2 Jupiter, Florida 33458 (561) 745-2534
Aero-Flex Corporation 3147 Jupiter Park Circle Suite 2 Jupiter, Florida 33458 (561) 745-2534 QUALITY WITHOUT QUESTION Flexible Metal Hose Assemblies Corrugated Metal Hoses that we offer: Hose Master Hoses OmegaFlex Hoses Penflex Hoses ANNUFLEX 700 Series Abrasion Resistant Tubular Braid for 1SBX Braid for 700 Series Hose Annuflex 700 Series Series 300 Hose- Stainless Steel Bronze Braid T304 Bronzeflex 1100 Series Monel Braid Bronze Tubular Braid for Series 400 ChemKing™ Hose Series 400 Stainless Steel Hose Monel Tubular Braid for Series 500 Series 600 Stainless Steel Braid ChlorSafe™ Hose Series 600 Stainless Steel Hose Series 100 - Helical, Stainless Steel, Series 700 Stainless Steel and Extraflex 9000 Series Standard Pitch Hose Compressed Hose Formaflex 900 Series Series 300 - Annular, Stainless Steel, Series 740 Monel Hose Standard Pitch Hose Series 794 Bronze Hose Hydraflex 9400 Series Series 400 - Annular, Bronze, Standard Pitch Hose Series 800 High Pressure Braid Interflex Series 800 Stainless Steel Hose Series 500 - Annular, Monel, Standard Pitch Hose Series 900 High Pressure Braid Masterflex 500 Series Series 900 Stainless Steel Hose Series 800 - Annular, Stainless Steel, Pressureflex HP High Pressure Hose and Braid Series P3 Stainless Steel Braid PressureMax HP Series P3 Stainless Steel Hose Tubular Braid for Series 100 Hose - Stainless Steel T304, T321 and T316 Stainless Steel Braid for Series Tar and Asphalt 400 Helical Hose Tubular Braid for Series 300 Stainless Steel Standard Braid for Ultraflex Braided Braid for Series 300 Series 700 Hose 1/4 - 12 inch 304, 316, & 321 Stainless Steel Single or Double Braid Why Flex Hose? It is comparatively light weight, temperature resistance, has great pressure retention, and allows for variance in fit up and alignment. -

Copper Pitting and Brass Dezincification
INSIGHTS INTO NON-UNIFORM COPPER AND BRASS CORROSION IN POTABLE WATER SYSTEMS Emily Allyn Sarver Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Civil and Environmental Engineering Dr. Marc Edwards, Committee Chair Dr. Sean Corcoran, Member Dr. Andrea Dietrich, Member Dr. Gerald Luttrell, Member Dr. Jeffrey Parks, Member November 1, 2010 Blacksburg, VA Keywords: copper pitting, brass dezincification, brass lead leaching, non-uniform corrosion, pipe-loop testing, corrosion inhibitors Copyright 2010, Emily A. Sarver INSIGHTS INTO NON-UNIFORM COPPER AND BRASS CORROSION IN POTABLE WATER SYSTEMS Emily Allyn Sarver ABSTRACT Non-uniform corrosion of copper and brass in potable water systems poses both economic and environmental problems associated with premature plumbing failures and release of metals. With respect to copper pitting corrosion, it was found that forensic testing (i.e., in pipe-loops) is the only investigative technique that can closely mimic conditions found in real water systems and produce unambiguous results; and, if used in combination with electrochemical techniques, it may also provide some mechanistic insights into the pitting process. Using pipe-loops, it was demonstrated that copper pitting in aggressive water qualities (i.e., chlorinated, high pH and low alkalinity) is deterministic and reproducible. Additionally, the effects of various chemical and physical factors on pitting were investigated. Overall, increased flow velocity and frequency, increased chlorine residual and decreased hardness were found to accelerate pitting; whereas increased phosphate and silica were found to decelerate pitting. Several mitigation strategies for copper pitting in aggressive water were further investigated, and experimental data were interpreted utilizing electrochemical theory to evaluate specific effects on the initiation and propagation phases of pitting. -

Outside Diameter Initiated Stress Corrosion Cracking Revised Final White Paper", Developed by AREVA and Westinghouse Under PWROG Program PA-MSC-0474
Program Management Office PWROG 102 Addison Road Connecticut 06095 q .Windsor, ko'ners-- PA-MSC-0474 Project Number 694 October 14, 2010 OG-10-354 U.S. Nuclear Regulatory Commission Document Control Desk Washington, DC 20555-0001 Subject: PWR Owners Group For Information Only - Outside Diameter Initiated Stress Corrosion Crackin2 Revised Final White Paper, PA-MSC-0474 This letter transmits two (2) non-proprietary copies of PWROG document "Outside Diameter Initiated Stress Corrosion Cracking Revised Final White Paper", developed by AREVA and Westinghouse under PWROG program PA-MSC-0474. This document is being submitted for information only. No safety evaluation (SE) is expected and therefore, no review fees should be incurred. This white paper summarizes the recent occurrences of ODSCC of stainless steel piping identified at Callaway, Wolf Creek and SONGS. Available operating experience (OE) at units that have observed similar phenomena is summarized as well to give a better understanding of the issue. Each of the key factors that contribute to ODSCC was considered along with the reported operating experience to perform a preliminary assessment of areas that may be susceptible to ODSCC. Also a qualitative evaluation was performed to determine whether ODSCC of stainless steel piping represents an immediate safety concern. This white paper is meant to address the issue of ODSCC of stainless steel systems for the near term. I&E guidelines are currently being developed through the PWROG to provide long term guidance. Correspondence related to this transmittal should be addressed to: Mr. W. Anthony Nowinowski, Manager Owners Group, Program Management Office Westinghouse Electric Company 1000 Westinghouse Drive, Suite 380 Cranberry Township, PA 16066 U.S. -

Elemental Fluorine Product Information (Pdf)
Elemental Fluorine Contents 1 Introduction ............................................................................................................... 4 2.1 Technical Application of Fluorine ............................................................................. 5 2.2 Electronic Application of Fluorine ........................................................................... 7 2.3 Fluorine On-Site Plant ............................................................................................ 8 3 Specifications ............................................................................................................ 9 4 Safety ...................................................................................................................... 10 4.1 Maintenance of the F2 system .............................................................................. 12 4.2 First Aid ................................................................................................................ 13 5.1 Chemical Properties ............................................................................................. 14 5.2 Physical Data ....................................................................................................... 15 6 Toxicity .................................................................................................................... 18 7 Shipping and Transport ........................................................................................... 20 8 Environment ........................................................................................................... -

GALVANIC/DISSIMILAR METAL CORROSION What It Is and How to Avoid It ASSDA Technical FAQ No 1 1 Edition 2, May 2009
AUSTRALIAN STAINLESS STEEL DEVELOPMENT ASSOCIATION GALVANIC/DISSIMILAR METAL CORROSION What it is and how to avoid it ASSDA Technical FAQ No 1 1 Edition 2, May 2009 Contact between dissimilar metals Metal to metal contact The graph shows that stainless steels have occurs frequently but is often not two ranges of potential. The usual, passive Galvanic corrosion can only occur if the a problem. The aluminium head behaviour is shown by the light hatching. dissimilar metals are in electrical contact. However, if the passive film breaks down, on a cast iron block, the solder on The contact may be direct or by an the stainless steel corrodes and its a copper pipe, galvanising on a external pipe or wire or bolt. If the potential is in the dark bar range. steel purlin and the steel fastener dissimilar metals are insulated from each in an aluminium sheet are common other by suitable plastic strips, washers or As a rule of thumb, if the potential examples. sleeves then galvanic corrosion cannot difference is less than 0.1 volt, then it is occur. Paint is not a reliable electrical unlikely that galvanic corrosion will be significant. WHAT CAUSES GALVANIC insulator especially under bolt heads or CORROSION? nuts or washers or near edges of sheets of If all three conditions are met then metal. The paint is usually damaged on galvanic corrosion is probable and the rate For galvanic or dissimilar or installation or by subsequent movement. of corrosion will be influenced by the electrolytic corrosion to occur, Note that the chromium oxide film layer relative area and the current density three conditions must be met: on the stainless steel is very thin and not delivered by the noble metal. -

Forms of Corrosion Uniform Corrosion
Chapter 4 Forms of corrosion Uniform corrosion In such type of corrosion there is a uniform decrease in the volume of the metal due to direct contact with the surrounding environment. Examples Dissolvation of Zn, Al metal in acids and Pb metal in base. Atmospheric corrosion Atmospheric corrosion take place due to direct contact between metal and atmosphere Types of atmosphere: Gas type: contain mainly oxygen gas( 23% W / W) and water vapor. Liquid type: contain mainly water and dissolved oxygen. iron rust Atmospheric corrosion involve formation of metal oxide layer when metal in direct contact with its surrounding environment. Example: Formation of iron rust due to contact with air Fe + air, H2O Fe(OH)2 Fe2O3 Fe2O3 .3H2O is Iron rust (reddish brown ppt.) Oxide layers Protective Non Protective Non active active Non porous porous Ex: Zn, Al, Ni Ex: iron rust Rate of atmospheric corrosion depends on Nature of metal or alloy. Nature of oxide layer. Factors affecting atmospheric corrosion: Type of metal or alloy. Type of atmosphere (dust, humidity, pollutant. Water vapor, gases,…) Temperature( as T increase as rate increase) Time: The extent of corrosion increases with increased time Surface Condition: more roughness, more corrosion The presence of foreign matter influence the speed of corrosion PLAY ACTIVE FIGURE Galvanic corrosion Take place due to the contact between bimetallic couple exposed to the same environment due to the potential difference. Mechanism of galvanic corrosion: For two different metals M1 and M2 when exposed to the same corrosive environment, if M1 is more active than M2 M1 M2 Anodic reaction: M M n+ + ne- (n=1, 2, 3, …) Anodic metal will be corroded. -

Introduction to Corrosion Your Friendly TSC Corrosion Staff
Introduction to Corrosion Your Friendly TSC Corrosion Staff: Daryl Little Ph.D. Materials Science & Engineering [email protected] 303-445-2384 Lee Sears Ph.D. Materials Science & Engineering [email protected] 303-445-2392 Jessica Torrey Ph.D. Materials Science & Engineering [email protected] 303-445-2376 Roger Turcotte, PE, CPS Materials Engineer [email protected] 303-445-2383 What is Corrosion? CORROSION IS DEFINED AS THE DETERIORATION OF A MATERIAL AND/OR ITS PROPERTIES CAUSED BY A REACTION WITH ITS ENVIRONMENT. Why is Corrosion a Major Concern? First and Most Important- Public Safety! • June 28, 1983: Mianus River Bridge, Greenwich, CT (TIME photo archive) • Northbound 3-lane section of I-95 bridge collapsed, killing 3 people • “Pin and Hanger” design was compromised when pin was displaced due to extensive corrosion of load-bearing components, amplified by salt from road maintenance. • Inadequate maintenance procedures were also cited as a contributing factor. • Subsequent to this failure, extensive inspections were conducted on this type of bridge across the country, and design modification were made to prevent catastrophic failures of this kind. First and Most Important- Public Safety! • April 28,1988, Aloha Airlines 737 Accident • 18 feet of cabin skin ripped off, resulting in the death of a flight attendant and many injuries • Attributed to corrosion fatigue Reclamation Case Study- Folsom Dam • Spillway gate No. 3 was damaged beyond repair, but no flooding occurred. • Replacement of No. 3 and repair of other gates cost ~$20 million and required 40% drainage of reservoir • Cause of failure: Increasing corrosion at the trunnion pin-hub interface raised the coefficient of friction and, therefore, the bending stress in the strut and the axial force in the brace exceeded limits. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Stainless Steel Selection Guide
TAContentsBLE OF Discovery of Stainless Steel . .page 2 What is Stainless Steel . .page 3 Stainless Steel Classifications . .page 4 • Austenitic . .page 4 • Ferritic . .page 5 • Duplex . .page 5 • Martensitic . .page 6 • Precipitation Hardening . .page 6 Nickel Based Alloys . .page 7 Strength & Heat Treatment . .page 7 The Basics of Corrosion . .page 8 • General or Uniform Corrosion . .page 9 • Galvanic Corrosion . .page 11 • Pitting Corrosion . .page 11 • Crevice Corrosion . .page 13 • Intergranular Corrosion . .page 14 • Stress Corrosion Cracking . .page 15 • Microbiologically Influence Corrosion . .page 17 Welding Stainless Steel . .page 18 Alloy Selection . .page 22 Wrought Stainless Steel Composition . .page 24 Wrought Nickel Alloy Composition . .page 25 Stainless Steel and Nickel Alloy Filler Metal . .page 26 PR“It’sEF stainlessAC steel,E it shouldn’t rust” This is often the kind of statements heard from individuals when discussing a failure of process piping or equipment. This is also an indication of how little is actually understood about stainless steel and the applications where it is used. For years the food, beverage and pharmaceutical industries have used stainless steels in their process piping systems. Most of the time stainless steel components provide satisfactory results. Occasionally a catastrophic failure will occur. The purpose of the information contained within this document is to bring an understanding to stainless steel, it’s uses, and why it will fail under certain conditions. In the following pages we will discuss the different classes of stainless steel, heat treatment, corrosion, welding, and finally material selection. As with any failure, it is imperative the cause of the failure be identified before a proper fix can be recognized. -

The Corrosion of Iron and Its Alloys
UNIVERSITY OF ILLINOIS LIBRARY Class Book Volume ^ M rlO-20M -r - 4 'f . # f- f f > # 4 Tjh- |fe ^ lINlN^ ' ^ ^ ^* 4 if i i if i^M^'M^i^ H II I li H : : \ ; . tNP* i rHt . ; ^ * 41 W 4s T »f 4 ^ -f 4. f -4- * i 4 4 : * 1- 1 4 % 3**:- ->^> A 4- - 4- + * ,,4 * 4" T '* * 4 -f- ± f * * 4- 4 -i * 4 ^ 4* -f + > * * + 1 f * 4 # * .-j* * + * ^ £ - jjR ^^MKlF^ f ^ f- + f v * IT :/^rV :,HH':,* ^ 4 * ^ 4 -f- «f f 4- 4 4 ,,rSK|M| ; * 4 # ; || II I II II f || .- -fr 4* , «g* 4 I. ^ vmiM 4- THE CORROSION OF IRON AND ITS ALLOYS BY RUSSELL SAMUEL HOWARD THESIS FOR THE DEGREE OF BACHELOR OF SCIENCE IN CHEMISTRY IN THE COLLEGE OF SCIENCE OF THE UNIVERSITY OF ILLINOIS Presented June, 1910 UNIVERSITY OF ILLINOIS June 1 1910 THIS IS TO CERTIFY THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Russel Samuel Howard ENTITLED The Corrosion of Iron and It s Alloys IS APPROVED BY ME AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF Bachelor of Scienc e In Chemistry Instructor in Charge Approved: HEAD OF DEPARTMENT OF 167529 Digitized by the Internet Archive in 2013 ^ttp://archive.org/details/corrosionofironiOOhowa CORROSION OF IRON AND ITS ALLOYS. In nature everything is subject to deterioration, varying from those substances decomposed by light to those only decomposed by the strong- est agents. The deterioration of metals as effected by alloying is the sub- ject of this paper and perhaps .no subject is of more vital importance to the builder and interest to the chemist than this one of corrosion. -

INFLUENCE of Ph on the LOCALIZED CORROSION of IRON
BNL 39687 MIT/BNL-86-6 INFORMAL REPORT INFLUENCE OF pH ON THE LOCALIZED CORROSION OF IRON MIT/BNL-86-6 BNL—39687 DE87 010452 R. Webley and R. Henry Consultants: H. Isaacs and G. Cragnolino June 1986 BROOKHAVEN STATION SCHOOL OF CHEMICAL ENGINEERING PRACTICE MASSACHUSETTS INSTITUTE OF TECHNOLOGY R.O. SPROULL. DIRECTOR F.J. HRACH, ASSISTANT DIRECTOR DEPARTMENT OF APPLIED SCIENCE BROOKHAVEN NATIONAL LABORATORY ASSOCIATED UNIVERSITIES. INC. UPTON, LONG ISLAND, NEW YORK 11973 UNDER CONTRACT NO. DE-AC02-76CH00016 WITH THE UNITED STATES DEPARTMENT OF ENERGY MASTER DISTRIBUTION OF TMiS DOCUMENT IS UNLIMITED DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontrac- tors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or use- fulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency, contractor or subcontractor thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency, contractor or subcontractor thereof. ABSTRACT The influence of pH on the pitting corrosion of iron in chloride and sul- fate solutions was determined using two artificial pit apparatuses to obtain the pH near the surface of the pit bottom. -
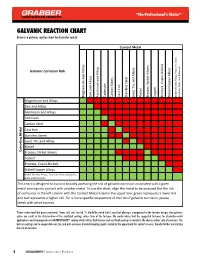
GALVANIC REACTION CHART Below Is a Galvanic Reaction Chart for Dissimilar Metals
GALVANIC REACTION CHART Below is a galvanic reaction chart for dissimilar metals. Contact Metal Silver, Silver, Galvanic Corrosion Risk Alloys Alloys Platinum Alloys Alloys, Titanium, and Alloys Steels Gold, and Iron Cast Stainless Steel Lead, Tin, and Magnesium and Zinc and Cadmium Carbon Nickel Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Aluminum Graphite, Nickel-Chrome Magnesium and Alloys Zinc and Alloys Aluminum and Alloys Cadmium Carbon Steel Cast Iron Metal Stainless Steels Lead, Tin, and Alloys Nickel Corroding Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Nickel-Chrome Alloys, Titanium, Silver, Graphite, Gold, and Platinum This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the Contact Metal listed in the upper row; green represents a lower risk and red represents a higher risk. For a more specific assessment of the risk of galvanic corrosion, please check with other sources. Please understand that green represents "lower risk" not "no risk." It should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. We would advise that the suggested fasteners for dissimilar-metal applications would incorporate our GRABBERGARD® coating which utilizes both barrier and sacrificial coatings to minimize the chance and/or rate of corrosion. The barrier coating used to encapsulate our zinc and anti-corrosion chemical bonding agents minimize the opportunity for contact to occur, thereby further minimizing the risk of corrosion.