The Roles of the Cockle Cerastoderma Edule L. on Ecosystem Functioning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -

Age Structure, Growth and Shell Form of Cerastoderma Glaucum (Bivalvia
Age structure, growth and shell form of Cerastoderma glaucum (Bivalvia: Cardiidae) from El Mellah lagoon, Algeria Lamia Bensaâd-Bendjedid, Saber Belhaoues, Asma Kerdoussi, Naouel Djebbari, Mardja Tahri, Mourad Bensouilah Laboratory of Ecobiology for Marine Environment and Coastlines, Faculty of Science, Badji Mokhtar University, Annaba 23000, Algeria. Corresponding author: L. Bensaâd-Benjedid, [email protected] Abstract. Cerastoderma glaucum (Poiret, 1789) the lagoon specialist cockle, represents one of the most abundant molluscs in El Mellah coastal lagoon (northeastern Algeria); several morphological and biological characteristics of this species were investigated over a period of 18 months (January 2014 to June 2015). Analysis of allometry and different morphometric indices indicate that this population tends to assume a globular shell shape which becomes elongated as the cockle grows. From length-frequency data examination using software FiSAT II and VONBIT for Excel, age was ranged from the first to the third year old, asymptotic length (L∞) was estimated to 48.061 mm and growth coefficient (K) was 0.71 -1 yr . The growth performance index (Φ′) and theoretical life span (tmax) were 3.21 and 4.23 yr respectively, total mortality rate (Z) obtained was 1.94 yr-1. Monthly variations in condition index were noticed, two sharp falls due spawning events were observed, the first spawning occurred between March and June and, the second one took place from August to October. The findings of the current study can be utilized to guide future research in various fields as pathology, biomonitoring and environmental management of this species. Key Words: Cerastoderma glaucum, shell shape, growth, Condition index, Algeria. -

University of Groningen Marine Benthic Metabarcoding
University of Groningen Marine benthic metabarcoding Klunder, Lise Margriet DOI: 10.33612/diss.135301602 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2020 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Klunder, L. M. (2020). Marine benthic metabarcoding: Anthropogenic effects on benthic diversity from shore to deep sea; assessed by metabarcoding and traditional taxonomy. University of Groningen. https://doi.org/10.33612/diss.135301602 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 26-12-2020 CHAPTER 2 Diversity of Wadden Sea macrofauna and meiofauna communities highest in DNA from extractions preceded by cell lysis Lise Klunder Gerard C.A. Duineveld Marc S.S. Lavaleye Henk W. van der Veer Per J. Palsbøll Judith D.L. van Bleijswijk Manuscript published in Journal of Sea Research 152 (2019) Chapter 2 ABSTRACT Metabarcoding of genetic material in environmental samples has increasingly been employed as a means to assess biodiversity, also of marine benthic communities. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -

Certification Report for Burry Inlet
Moody Marine Ltd Burry Inlet Cockle Fishery: Public Certification Report MOODY MARINE LTD Ref: 82001 v5 Author(s): T Holt, A Hough Certification Report for Burry Inlet Cockle Fishery Client: South Wales Sea Fishery Committee Certification Body: Client Contact: Moody Marine Ltd Director: Mr P Coates Moody International Certification South Wales Sea Fisheries Committee Salisbury House Queens Buildings Stephenson’s Way Cambrian Place Wyvern Business Park Swansea. SA1 1TW Derby. DE21 6LY UK UK Tel: +44 (0) 1704 834644 01792 654466 Fax: +44 (0) 1332 675152 01792 645987 FN 07/019 82001 v4 1 Moody Marine Ltd Burry Inlet Cockle Fishery: Public Certification Report CONTENTS 1. INTRODUCTION..................................................................................................................................... 3 1.1 THE FISHERY PROPOSED FOR CERTIFICATION...................................................................................... 3 1.2 REPORT STRUCTURE AND ASSESSMENT PROCESS .............................................................................. 3 1.3 INFORMATION SOURCES USED ............................................................................................................ 4 2 BACKGROUND TO THE FISHERY .................................................................................................... 6 2.1 BIOLOGY OF THE TARGET SPECIES ..................................................................................................... 6 2.2 HISTORY OF THE FISHERY ................................................................................................................. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Tayside, Central and Fife Tayside, Central and Fife
Detail of the Lower Devonian jawless, armoured fish Cephalaspis from Balruddery Den. © Perth Museum & Art Gallery, Perth & Kinross Council Review of Fossil Collections in Scotland Tayside, Central and Fife Tayside, Central and Fife Stirling Smith Art Gallery and Museum Perth Museum and Art Gallery (Culture Perth and Kinross) The McManus: Dundee’s Art Gallery and Museum (Leisure and Culture Dundee) Broughty Castle (Leisure and Culture Dundee) D’Arcy Thompson Zoology Museum and University Herbarium (University of Dundee Museum Collections) Montrose Museum (Angus Alive) Museums of the University of St Andrews Fife Collections Centre (Fife Cultural Trust) St Andrews Museum (Fife Cultural Trust) Kirkcaldy Galleries (Fife Cultural Trust) Falkirk Collections Centre (Falkirk Community Trust) 1 Stirling Smith Art Gallery and Museum Collection type: Independent Accreditation: 2016 Dumbarton Road, Stirling, FK8 2KR Contact: [email protected] Location of collections The Smith Art Gallery and Museum, formerly known as the Smith Institute, was established at the bequest of artist Thomas Stuart Smith (1815-1869) on land supplied by the Burgh of Stirling. The Institute opened in 1874. Fossils are housed onsite in one of several storerooms. Size of collections 700 fossils. Onsite records The CMS has recently been updated to Adlib (Axiel Collection); all fossils have a basic entry with additional details on MDA cards. Collection highlights 1. Fossils linked to Robert Kidston (1852-1924). 2. Silurian graptolite fossils linked to Professor Henry Alleyne Nicholson (1844-1899). 3. Dura Den fossils linked to Reverend John Anderson (1796-1864). Published information Traquair, R.H. (1900). XXXII.—Report on Fossil Fishes collected by the Geological Survey of Scotland in the Silurian Rocks of the South of Scotland. -
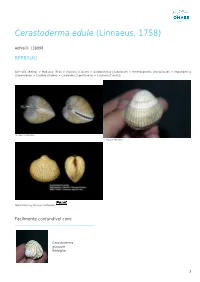
Cerastoderma Edule (Linnaeus, 1758)
Cerastoderma edule (Linnaeus, 1758) AphiaID: 138998 BERBIGÃO Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Cardiida (Ordem) > Cardioidea (Superfamilia) > Cardiidae (Familia) © Vasco Ferreira © Vasco Ferreira Natural History Museum Rotterdam Facilmente confundível com: Cerastoderma glaucum Berbigão 1 Estatuto de Conservação Sinónimos Cardium belgicum De Malzine, 1867 Cardium crenulatum Lamarck, 1819 Cardium edule Linnaeus, 1758 Cardium edule burchanae Girscher, 1938 Cardium edule var. batesoni Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. loppensi Mars, 1951 Cardium edule var. maculata Dautzenberg, 1890 Cardium edule var. major Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. mareotica Pallary, 1912 Cardium edule var. regularis Pallary, 1900 Cardium edule var. sibenicensis Brusina, 1870 Cardium mercatorium Coen, 1915 Cardium nunninkae Lucas, 1984 Cardium obtritum Locard, 1886 Cardium quadrarium Reeve, 1845 Cardium vulgare da Costa, 1778 Cardium vulgatum Tryon, 1872 Cerastoderma edule var. sinicola Lacourt, 1974 Cerastoderma nunninkae Lucas, 1984 Referências basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Poorten, J.J. ter, 2005. Outline of a systematic index – Recent Cardiidae (Lamarck, 1809). VISAYA net. (Updated 2009 for WoRMS), available online at http://www.conchology.be/en/shelltopics/visaya-net/date.php?year=2005 [details] 2 ecology source Coscia, I., P.E. Robins, J.S. Porter, S.K. Malham & J.E. Ironside. (2013). Modelled larval dispersal and measured gene flow: seascape genetics of the common cockle Cerastoderma edule in the southern Irish Sea. -

Ecological Studies in South Benfleet Creek with Special Reference to the Amphipod Genus Corophium B.A.*
Reprinted from, THE ESSEX NATURALIST for 1961 Ecological Studies in South Benfleet Creek with Special Reference to the Amphipod Genus Corophium B.A.* This paper is an account of the work done on some ecological aspects of South Benfleet Creek. A description of the Creek is given in the Introduction and a brief account of the methods used in the second section. The ecology of the most important species found in the Creek is described. The Creek is most interesting with regard to the distribution of the two species of Corophium found there, one of which, Corophium arenarium, is a relatively rare species. It has been found that the composition of the substratum, and associated factors such as water content, is the dominant factor in determining the distribution of the two species of Corophium and of the other two most important species, Nereis diversicolor and Scrobicularia plana. The import- ant distinctions between the habitat of Corophium volutator and Corophium arenarium have also been worked out. INTRODUCTION Benfleet Creek South Benfleet Creek (G.R. TQ 79 85) is a small tidal inlet on the north bank of the River Thames about 7 miles from its mouth at Shoeburyness. The Creek is just over 3 miles long, from Canvey Point to South Benfleet Bridge, nearly half a mile wide at its mouth and about 80 yards wide near the bridge. It runs in a S.S.E.-N.N.W. direction between the low-lying land of Canvey Island and Hadleigh Marsh. Because the surrounding land is all below H.W.M.M.T.* level, strong concrete embank- ments have been built along the whole length of the creek, on both banks. -

Occurrence of an Unusual Branchial Mycoplasma-Like Infection in Cockle Cerastoderma Edule (Mollusca, Bivalvia)
DISEASES OF AQUATIC ORGANISMS Vol. 16: 55-59.1993 Published June 24 Dis. aquat. Org. I Occurrence of an unusual branchial mycoplasma-like infection in cockle Cerastoderma edule (Mollusca, Bivalvia) Carlos Azevedo Department of Cell Biology, Institute of Biomedical Sciences, and IMAR - Institute of Marine Research, University of Oporto, Lg. A. Salazar no. 2, P-4000 Porto. Portugal ABSTRACT: Unusual cytoplasmic structures were observed and described in gill epithelial cells during an occurrence of high mortality of cockle Cerastoderma edule obtained from an estuarine region of central Portugal (Aveiro)in 1991 and 1992. Ultrastructural studies on gaping cockles revealed a gill in- fection characterized by the presence of numerous long rod-shaped structures (RSS) regularly distrib- uted, singly or in groups, within the cytoplasmic cisternae of gill epithelial cells. The RSS of 0.5 to 4 pm length and a uniform width of 0.9 pm presented a well organized structure in the basal portion of the host cell. Each RSS showed a homogeneous and electron-dense material delinuted by a tri-laminar membrane devoid of cell wall. Lysed ultrastructural aspects of the host cell were observed. Due to their ultrastructural organization these RSS were identified as mycoplasma-like microorganisms and their pathogenic activity in the mortality of the cockle is discussed. INTRODUCTION during the summer of 1991 and 1992 from an estuarine region of central Portugal (Aveiro). Parasitic and symbiotic species of prokaryotic micro- Small living fragments of the gills were firstly ob- organisms have been reported in a range of marine served by light microscopy (LM). For transmission animals (reviews by Tully & Whitcomb 1979, Lauckner electron microscopy (TEM) small fragments of the 1983, Sparks 1985). -

Biogeographic Patterns of the Marine Bivalve Cerastoderma Edule Along European Atlantic Coasts
Biogeographic patterns of the marine bivalve Cerastoderma edule along European Atlantic coasts DISSERTATION Zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrecht-Universität zu Kiel vorgelegt von Manuela Krakau Kiel 2008 Angefertigt an der Wattenmeerstation Sylt Stiftung Alfred-Wegener-Institut für Polar- und Meeresforschung Referent: Prof. Dr. Karsten Reise Koreferent: Prof. Dr. Reinhold Hanel Tag der mündlichen Prüfung: 10. Juli 2008 Zum Druck genehmigt: 10. Juli 2008 CONTENTS SUMMARY ...…...………………………………….………………………… I ZUSAMMENFASSUNG .……………………………………………………... III GENERAL INTRODUCTION .………………………………………………….. 1 CHAPTER 1: Shell forms of the intertidal bivalve Cerastoderma edule L. from Africa to the Arctic .………………………………………… 9 CHAPTER 2: Cockle parasites across biogeographic provinces ..……......… 36 CHAPTER 3: Genetic diversity in high latitudes – an intertidal bivalve contradicts a common pattern ..…………………………… 59 GENERAL DISCUSSION .…………………...……………………………….. 89 REFERENCES …..…………………………………………………………... 95 APPENDIX ………………………………………………………………… X1 ACKNOWLEDGEMENTS /D ANKSAGUNG Biogeographic patterns of the marine bivalve C. edule SUMMARY SUMMARY The cockle Cerastoderma edule is a common bivalve that inhabits the marine soft-bottom intertidal along European shores. This invertebrate plays a key role in coastal food webs of the Northeast Atlantic coasts due of its high abundances. I studied cockles from 19 sites along the distribution range with the aim to describe the variation of geographic population -

Life History of the Amphipod Corophium Insidiosum (Crustacea: Amphipoda) from Mar Piccolo (Ionian Sea, Italy)
SCIENTIA MARINA 70 (3) September 2006, 355-362, Barcelona (Spain) ISSN: 0214-8358 Life history of the amphipod Corophium insidiosum (Crustacea: Amphipoda) from Mar Piccolo (Ionian Sea, Italy) ERMELINDA PRATO and FRANCESCA BIANDOLINO Istituto Sperimentale Talassografico, I.A.M.C - C.N.R., Via Roma, 3, 74100 Taranto, Italy. E-mail: [email protected] SUMMARY: A one-year study was conducted on the life history of the amphipod Corophium insidiosum (Crawford, 1937) in the Mar Piccolo estuary (Southern Italy). Monthly collections were made to investigate certain aspects of population struc- ture, abundance and reproductive biology. Population density showed a clear seasonal variation: with a maximum in spring- summer and a minimum in autumn-winter. Although brooding females were present all year round, recruitment occurred in spring, decreased in summer, peaked in autumn and almost ceased during the winter. 7-8 new cohorts in all samples could be recognised from April 2002 to November 2002. Mean longevity was ~5 to 6 months, and the estimated lifespan was longer for individuals born in late summer than for individuals born in spring. The sex ratio favoured females with a mean value of 1.51, but males grew faster and attained a larger maximum body length than females. Males and females became distinguishable at roughly >2 mm, reaching a maximum size of 5.6 mm for females and 6.0 mm for males during the win- ter months. The females reproduced for the first time when they reached 2.2 mm body length. The number of eggs carried by females was related to the size of the female.