Using Multiple Sentinel Species and Stable Isotopes to Understand Mercury Sources and Fate in Temperate Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliographie Acadienne - Liste De Volumes, Brochures Et Thèses (Antérieur À 1976) - Table Des Matières 09-02-18 09:08
ACADIE - Bibliographie acadienne - liste de volumes, brochures et thèses (antérieur à 1976) - Table des matières 09-02-18 09:08 Bibliographie acadienne - liste de volumes, brochures et thèses (antérieur à 1976) Table des matières Préface Introduction Abréviations Bibliographie Annexe Liste sélective de bibliographies, incluant des titres susceptibles d'intéresser les chercheurs sur l'Acadie Index auteurs-titres : A - D E - I J - M N - O P - Z Index sujets : A - L M - Y http://www0.umoncton.ca/etudeacadiennes/centre/guide/tabmat-3.html Page 1 of 1 ACADIE - Bibliographie acadienne (antérieur à 1976) 09-02-18 09:08 Bibliographie acadienne Liste de volumes, brochures et thèses concernant L'Acadie et les Acadiens Rédigée sous la direction du R.P. Anselme Chiasson Directeur du Centre d'études acadiennes Compilée par Claude Guilbeault (Droits réservés) Centre d'études acadiennes Université de Moncton 1976 PRÉFACE Le Centre d'études acadiennes de l'Université de Moncton cherche à accumuler toute la documentation manuscrite ou imprimée qui concerne les Acadiens. Son but est aussi de faciliter la recherche aux chercheurs en mettant à leur disposition toute cette documentation et les instruments nécessaires à son accessibilité. Dans ce sens, le Centre publiait en 1975 un inventaire général des archives publiques ou semi-publiques concernant les Acadiens. L'accueil fait à ce volume par les historiens et les professeurs d'histoire indique clairement qu'il répondait à un besoin manifeste. Cet inventaire n'était que le premier d'une série de travaux que le Centre se proposait de rédiger. D'autres devaient suivre, tels une bibliographie acadienne, un dictionnaire généalogique, un inventaire des articles de revue, une brochure sur le folklore, etc. -

Bulletin D'information 2019
Fall | Automne 2019 New Brun und Fo swick Wildlife Trust F swick nds en Fidu eau-Brun cie pour la Faune du Nouv Header photo: Cody Pytlak New Brunswick Conservation plates Les Plaques « Conservation » du Nouveau-Brunswick onservation plates have become es plaques d’immatriculation a staple in New Brunswick. L« Conservation » sont devenues C communes au Nouveau-Brunswick. You can see them on just about On en voit à pratiquement chacun de any drive. The selection of plates nos déplacements. Quatre options de includes: the Atlantic salmon, the plaques sont actuellement offertes : white tailed deer, the purple violet le saumon de l’Atlantique, le cerf and the black-capped chickadee. de Virginie, la violette cuculée et la mésange à tête noire. This year, 89 local non-governmental organizations and community Cette année, 89 organismes non gouvernementaux ou groupes groups have received funding to communautaires ont reçu un undertake 131 projects. financement qui leur ont permis d’entreprendre 131 projets. The projects have included new and ongoing work in fisheries, Ceux-ci concernaient des initiatives wildlife, trapping, biodiversity and nouvelles ou en cours portant sur la pêche, la faune, le piégeage, la conservation education. biodiversité et la formation en matière de conservation. The Miramichi Salmon Association is a big supporter of the plates! They La Miramichi Salmon Association est have been purchasing Conservation une grande partisane de ces plaques! Ses membres achètent les plaques plates for their vehicles since they « Conservation » pour leurs véhicules started being produced. depuis qu’elles existent. “By getting a conservation plate (we « En vous procurant une plaque recommend the salmon!) you help “Conservation” (nous recommandons support our MSA Field Programs. -

New Brunswick Wildlife Trust Fund List of Projects Approved February 2019
NEW BRUNSWICK WILDLIFE TRUST FUND LIST OF PROJECTS APPROVED FEBRUARY 2019 NB Wildlife Federation Adopt – A - Stream $4,500. Restigouche River Watershed Management Council Inc. Atlantic Salmon Survey 2019 – Restigouche River System $7,000. Bassins Versants de la Baie des Chaleurs Buffer Zones Monitoring and Restoration $5,500. Nepisiguit Salmon Association Nepisiguit Salmon Association Salmon Enhancement Project $9,000. Pabineau First Nation Little River Smolt Survey – 2019 $10,000. Partenariat pour la gestion intégrée du bassin versant de la baie de Caraquet Inc. Assessment of the Streams Flowing into the Caraquet River $6,000. Comité Sauvons nos Rivières Neguac Inc. Ecological Restoration of Degraded Aquatic Habitats in the McKnight Brook $15,000. Miramichi Salmon Association Inc. Striped Bass Spawning Survey 2019 $12,000. Miramichi Salmon Association Inc. Restoring Critically Important Atlantic Salmon Habitat – Government Pool, SW Miramichi River $12,000. Miramichi Watershed Management Committee Miramichi Lake Smallmouth Bass Containment 2019 $12,000. Atlantic Salmon Federation Miramichi Atlantic Salmon Tracking $15,000. Dr. Charles Sacobie, UNB Hypoxia and Temperature Tolerance of Brook Trout (Salvelinus fontinalis) Populations in two Distinct Thermal Regimes in the Miramichi Watershed $10,000. Dr. Wendy Monk, Canadian Rivers Institute, UNB Effects of Warming on Freshwater Streams in New Brunswick: A whole ecosystem study using DNA metabarcoding and trait-based food webs $8,000. Les Ami (e) s de la Kouchibouguacis Inc. Salmon Population Restoration to the Kouchibouguacis River 2019 $9,000. Shediac Bay Watershed Association Fish Habitat Restoration, Evaluation and Education for the Enhancement of Salmonid Populations in the Shediac Bay Watershed $8,000. Dr. Alyre Chiasson, Université de Moncton The Effects of Extreme Oscillations in Water Temperature on Survival of Brook Trout in the Petitcodiac Watershed, a within Stream Study $5,000. -

Annual Report 1958-59-, Which Also Contains Lists of Its Scientific Staff and Various Publications
LII3RA17.Y NS C(.1;772,, ,-)011 V,IEST Fte 24 3 Fjp,o.:ay:s CANADA OTTSWA, Or..;`1.2.:210, 1(1A. Wi7,6 e, ‘-;/- N 1,101te _Ale)OCEe3 Vet VISI-e'PeS 11,0011. esràzs s.rr., 240 Oefe.10i CeA.D.A. • • .. 'UPI. 0E6 , s.. , Being the Ninety-second Annual Fisheries Report of the Government of Canada ERRATA Department of Fisheries of Canada, Annual Report, 1958-59. Page 32, paragraph 1, line 4, the figure '1960' should read '1959 1 . Page 51, paragraph 1, line 8, the figure '1947' should read '1957'. 78047-8--1 4 THE QuEEN's PRINTER AND CONTROLLER OF STATIONERY OTTAWA, 1960 Price 50 cents. Cat. No. Fs. 1-59 To His Excellency Major-General Georges P. Vanier, D.S.O., M.C., C.D., Governor General and Commander-in-Chief of Canada. May it Please Your Excellency : I have the honour herewith, for the information of Your Excellency and the Parliament of Canada, to present the Annual Report of the Department of Fisheries for the fiscal year 1958-1959. Respectfully submitted Minister of Fisheries. 78047-8--1I 4 To The Honourable J. Angus MaCLean, M.P., Minister of Fisheries, Ottawa, Canada. Sir: I submit herewith the Annual Report of the Department of Fisheries for the fiscal year 1958-1959. I have the honour to be, Sir, Your obedient servant e,6) • Deputy Minister. CONTENTS Page Introduction 7 Conservation and Development Service 10 Departmental Vessels 29 Inspection and Consumer Service 32 Economics Service 44 Information and Educational Service 46 Industrial Development Service 49 1 Fishermen's Indemnity Plan 51 Fisheries Prices Support Board 53 Fisheries Research Board of Canada 56 International Commissions 70 Special Committees 92 The Fishing Industry 93 Statistics of the Fisheries 99 APPENDICES 1. -

Maritime Provinces Fishery Regulations Règlement De Pêche Des Provinces Maritimes TABLE of PROVISIONS TABLE ANALYTIQUE
CANADA CONSOLIDATION CODIFICATION Maritime Provinces Fishery Règlement de pêche des Regulations provinces maritimes SOR/93-55 DORS/93-55 Current to September 11, 2021 À jour au 11 septembre 2021 Last amended on May 14, 2021 Dernière modification le 14 mai 2021 Published by the Minister of Justice at the following address: Publié par le ministre de la Justice à l’adresse suivante : http://laws-lois.justice.gc.ca http://lois-laws.justice.gc.ca OFFICIAL STATUS CARACTÈRE OFFICIEL OF CONSOLIDATIONS DES CODIFICATIONS Subsections 31(1) and (3) of the Legislation Revision and Les paragraphes 31(1) et (3) de la Loi sur la révision et la Consolidation Act, in force on June 1, 2009, provide as codification des textes législatifs, en vigueur le 1er juin follows: 2009, prévoient ce qui suit : Published consolidation is evidence Codifications comme élément de preuve 31 (1) Every copy of a consolidated statute or consolidated 31 (1) Tout exemplaire d'une loi codifiée ou d'un règlement regulation published by the Minister under this Act in either codifié, publié par le ministre en vertu de la présente loi sur print or electronic form is evidence of that statute or regula- support papier ou sur support électronique, fait foi de cette tion and of its contents and every copy purporting to be pub- loi ou de ce règlement et de son contenu. Tout exemplaire lished by the Minister is deemed to be so published, unless donné comme publié par le ministre est réputé avoir été ainsi the contrary is shown. publié, sauf preuve contraire. ... [...] Inconsistencies in -

Social Studies Grade 3 Provincial Identity
Social Studies Grade 3 Curriculum - Provincial ldentity Implementation September 2011 New~Nouveauk Brunsw1c Acknowledgements The Departments of Education acknowledge the work of the social studies consultants and other educators who served on the regional social studies committee. New Brunswick Newfoundland and Labrador Barbara Hillman Darryl Fillier John Hildebrand Nova Scotia Prince Edward Island Mary Fedorchuk Bethany Doiron Bruce Fisher Laura Ann Noye Rick McDonald Jennifer Burke The Departments of Education also acknowledge the contribution of all the educators who served on provincial writing teams and curriculum committees, and who reviewed and/or piloted the curriculum. Table of Contents Introduction ........................................................................................................................................................ 1 Program Designs and Outcomes ..................................................................................................................... 3 Overview ................................................................................................................................................... 3 Essential Graduation Learnings .................................................................................................................... 4 General Curriculum Outcomes ..................................................................................................................... 6 Processes .................................................................................................................................................. -

Grade 3 Social Studies That Have Been Organized According and Perspectives to the Six Conceptual Strands and the Three Processes
2012 Prince Edward Island Department of Education and Early Childhood Development 250 Water Street, Suite 101 Summerside, Prince Edward Island Canada, C1N 1B6 Tel: (902) 438-4130 Fax: (902) 438-4062 www.gov.pe.ca/eecd/ CONTENTS Acknowledgments The Prince Edward Island Department of Education and Early Childhood Development acknowledges the work of the social studies consultants and other educators who served on the regional social studies committee. New Brunswick Newfoundland and Labrador John Hildebrand Darryl Fillier Barbara Hillman Nova Scotia Prince Edward Island Mary Fedorchuk Bethany Doiron Bruce Fisher Laura Ann Noye Rick McDonald Jennifer Burke The Prince Edward Island Department of Education and Early Childhood Development also acknowledges the contribution of all the educators who served on provincial writing teams and curriculum committees, and who reviewed or piloted the curriculum. The Prince Edward Island Department of Education and Early Childhood Development recognizes the contribution made by Tammy MacDonald, Consultation/Negotiation Coordinator/Research Director of the Mi’kmaq Confederacy of Prince Edward Island, for her contribution to the development of this curriculum. ATLANTIC CANADA SOCIAL STUDIES CURRICULUM GUIDE: GRADE 3 i CONTENTS ii ATLANTIC CANADA SOCIAL STUDIES CURRICULUM GUIDE: GRADE 3 CONTENTS Contents Introduction Background ..................................................................................1 Aims of Social Studies ..................................................................1 Purpose -

Graphs on the Placer-Nomenclature, Cartography, Historic Sites, Boundaries and Settlement- Origins of the Province of L^Ew Brunswick
FROM THE TRANSACTIONS OF THE ROYAL SOCIETY OF CANADA SECOND SERIES—1906-1907 VOLUME XII SECTION II ENGLISH HISTORY, LITERATURE, ARCHEOLOGY, ETC. Additions and Corrections to Mono graphs on the Placer-Nomenclature, Cartography, Historic Sites, Boundaries and Settlement- origins of the Province of l^ew Brunswick By W. F. GANONG, M.A., Ph.D. FOR SALE BY J. HOPE & SONS, OTTAWA; THE COPP-C^I^K CO., TORONTO BERNARD QUARITCH, LONDON, ENGLAND 1906 SECTION II., 1906. [ 8 J TRANS. R. S. C. I.—Additions and Correciions to Monographs on the Place-nomenclature, Cartography, Historic Sites, Boundaries and Settlement- origins of the Province of New Brunswich. (Contributions to the History of New Brunswick, No. 7.) BY W. F. GANONG, M.A., PH.D. (Communicated by Dr. S. E. Dawson.) I.—Additions and Corrections to the Plan for a General History of New Brunswick, 11.—^Additions and Corrections to the Monograph on Place-nomenclature. ^ IHr-g::45ditions and Corrections to the Monograph on'Cartography. IV.—^Additions arTd-Coxrecfions to the Monograph on Historic Sites. V.—^Additions and Corrections to the-MQnograph on Evolution of Boundaries. VI.—^Additions an^d Corrections to the Monograph on "^Settle^ent-Origins. Title-page and Contents to the series. The five monograplis of this series were designed to cover the hifetorical geography of IsTew Brunswick, and in plan at least they dc» so. The organization given the respective subjects by their publica tion has had the result not only of directing my own studies further, but also of bringing much additional information from correspondents. Thus a large amount of new material and some corrections have come into my hands, and it is the object of this work to present them, and in such a way that all items may be referred to their proper places in the respective monographs. -

Status of Atlantic Salmon in Salmon Fishing Area 15, Gulf Region, New Brunswick, 199 4
Not to be cited without Ne pas citer sans permission of the authors' autorisation des auteurs ' DFO Atlantic Fisheries MPO Pêches de l'Atlantique Research Document 95/87 Document de recherche 95/8 7 STATUS OF ATLANTIC SALMON IN SALMON FISHING AREA 15, GULF REGION, NEW BRUNSWICK, 199 4 by A . Locke, R . Pickard, F . Mowbray, A . Madden2, and P . Cameron Department of Fisheries and Oceans Science Branch, Gulf Regio n P .O . Box 503 0 Moncton, New Brunswick E1C 9B 6 2New Brunswick Department of Natural Resources and Energy P .O . Box 277, Campbellto n New Brunswick E3N 3G 4 'This series documents the 'La présente série documente scientific basis for the les bases scientifiques des evaluation of fisheries évaluations des ressources resources in Atlantic Canada . halieutiques sur la côte As such, it addresses the atlantique du Canada . Elle issues of the day in the time traite des problèmes courants frames required and the selon les échéanciers dictés . documents it contains are not Les documents qu'elle contient intended as definitive ne doivent pas être considérés statements on the subjects comme des énoncés définitifs addressed but rather as sur les sujets traités, mais progress reports on ongoing plutôt comme des rapports investigations . d'étape sur les études en cours . Research documents are produced Les Documents de recherche sont in the official language in publiés dans la langue which they are provided to the officielle utilisée dans le secretariat . manuscrit envoyé au secrétariat . Table of content s Abstract . 3 Summary sheet . 4 1 - Introduction . 5 2 - Description of fisheries . 5 3 - Fishery data . -
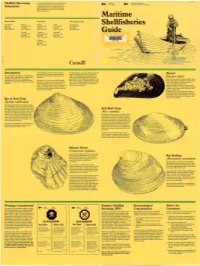
Shellfish Harvesting Information
Shellfish Harvesting It is very important before you col lect any shell Fishe ries Environmen t Canada fish that you ensure the area is not closed . and Oceans Environmental Protection Service Information A check is as simple as using the local tele phone directory to find the federal fisheries •• •• office nearest you or ca ll one of the central offices listed below. Maritime New Brunswick Nova Scotia Prince Edward Island St. Andrews Moncion Sydney Halifax Charlottetown Shellfisheries Box 210 P.O . Box 5030 P.O. Box 1085 P 0 . Box 550 P.O. Box 1236 EOG 2XO E1C 9B6 B1 p 6J7 B3J 2S7 C1A 7M8 (506) 529-8847 (506) 758-9044 (902) 564-7276 (902) 426-24 73 (902) 566-7800 Tracadie Yarmouth Antigonish Guide .,. P.O. Box 1670 215 Main St reet P.O. Box 1183 EOC 2BO B5A 1C6 B2G 2M5 (506) 395-6321 (902) 742-9122 (902) 863-5670 Liverpool P.O. Box 190 BOT 1KO (902) 354-3459 Canada Introduction Fresh shellfish can be purchased at any of As with an y food , care must be taken to ensure hundreds of fish markets , and are served at th at the shellfish gathered are not contam Mussel One of the great attractions of Canada's Mari restaurants and snack bars throughout the inated. This guide has been prepared to pro time provinces is the selection of seafood del Maritimes. vide the recreational digger with information (Mytilus edulis) icacies to be enjoyed here. Among the pertaining to the safe harvest of shellfish in the Rocky shores along the three provinces' coast favourites are the many varieties of shellfish In search of a recreational outing ? Shellfish Maritimes. -

Lovell's Gazetteer of the Dominion of Canada. 347
, LOVELL'S GAZETTEER OF THE DOMINION OF CANADA. 347 (Roman Catholic and Presbyterian), 3 stores, There Is a post office at Cache Creek. The dis- 1 temperance hotel. 1 saw mill, and 1 box fac- trict is almost entirely devoted to agricul- tory, besides telegraph and express office. ture, some of the best farms in the province Pop. fluctuating, average about 650. being found here. BYRNEDALE. a post settlement In Essex CACHE LAKE, a small post office and flag CO., 5J miles from Belle River on the Great station on the C.P.R., im Thunder Bay district, Western dijvislon of the G.T.R. Pop. 200. northwest Ont. Pop. 10. BYRON, a post village in Middlesex co., CACHE LAKE, in Nipissing dist., Ont , near Ont., on the River Thames. 5 miles from Hyde the entrance to Algonquin National Park and Springbank Line, Park Station, terminus of close to the line of the Ottawa and Parry London Electric Railway. It has good water- Sound branch of the G.T.R. power privileges and contains 1 flour mill, 2 CACHE LAKE, in Badarerow t'p.. dist. of Ni- stores, 2 churches and 1 telephone office. The T^-tpsing. N. Ont.. lyitis: south of the Sturgeon largest single span steel bridge in the Province River and to the northwest of Cache Bay sta- is In course of erection here. Pop. 100. tion on the Labrador, C.P.R., on an inlet of Lake Nipis- BYRON BAY, on the east coast of sing. 50° 40' north, long. 50° 30' west. It is near lat. -

New Brunswick Wildlife Trust Fund List of Projects 2019
NEW BRUNSWICK WILDLIFE TRUST FUND LIST OF PROJECTS 2019 NB Wildlife Federation Adopt – A - Stream $4,500. Restigouche River Watershed Management Council Inc. Atlantic Salmon Survey 2019 – Restigouche River System $7,000. Bassins Versants de la Baie des Chaleurs Buffer Zones Monitoring and Restoration $5,500. Nepisiguit Salmon Association Nepisiguit Salmon Association Salmon Enhancement Project $9,000. Pabineau First Nation Little River Smolt Survey – 2019 $10,000. Partenariat pour la gestion intégrée du bassin versant de la baie de Caraquet Inc. Assessment of the Streams Flowing into the Caraquet River $6,000. Comité Sauvons nos Rivières Neguac Inc. Ecological Restoration of Degraded Aquatic Habitats in the McKnight Brook $15,000. Miramichi Salmon Association Inc. Striped Bass Spawning Survey 2019 $12,000. Miramichi Salmon Association Inc. Restoring Critically Important Atlantic Salmon Habitat – Government Pool, SW Miramichi River $12,000. Miramichi Watershed Management Committee Miramichi Lake Smallmouth Bass Containment 2019 $12,000. Atlantic Salmon Federation Miramichi Atlantic Salmon Tracking $15,000. Dr. Charles Sacobie, UNB Hypoxia and Temperature Tolerance of Brook Trout (Salvelinus fontinalis) Populations in two Distinct Thermal Regimes in the Miramichi Watershed $10,000. Dr. Wendy Monk, Canadian Rivers Institute, UNB Effects of Warming on Freshwater Streams in New Brunswick: A whole ecosystem study using DNA metabarcoding and trait-based food webs $8,000. Les Ami (e) s de la Kouchibouguacis Inc. Salmon Population Restoration to the Kouchibouguacis River 2019 $9,000. Shediac Bay Watershed Association Fish Habitat Restoration, Evaluation and Education for the Enhancement of Salmonid Populations in the Shediac Bay Watershed $8,000. Dr. Alyre Chiasson, Université de Moncton The Effects of Extreme Oscillations in Water Temperature on Survival of Brook Trout in the Petitcodiac Watershed, a within Stream Study $5,000.