Double Outlet Right Atrium with Three Atrioventricular Valves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Anatomy of the Heart
Anatomy of the Heart DR. SAEED VOHRA DR. SANAA AL-SHAARAWI OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle. -

The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S
The Biomechanical Puncture Study of the Fossa Ovalis in Human and Porcine Hearts Daniel S. Balto, Steven A. Howard, BMEn., Paul A. Iaizzo, Ph.D.2 Departments of Biomedical Engineering1 and Surgery2 University of Minnesota, Minneapolis, MN 55455 Introduction Apparatus Conclusion Approximately 1 in 4 humans on this earth is born with a congenital heart •The apparatus designed for this study was great for initial testing of the defect that involves the failure of the complete formation of the fossa ovalis tissue properties of the fossa ovalis of large mammalian hearts, either of membrane between the left and right atria1. This defect, better known as a PFO research animals or of human donor hearts. However, physiological or Patent Fossa Ovalis is an opening in the fossa ovalis membrane that results properties weren’t really observed in the testing and so that is a starting from the failure of the fetal structures (septum primum and septum secundum) point for advancing the quality of this research. The ability for the system to from closing together at the moment of birth2. The opening can be classified in have interchangeable depression, puncture, and dilation devices adds to the a variety of ways, but the most common are “shunts” which cause blood to flexibility of the current setup. The visualization of the inside of the heart flow from one atria to the other. A shunt that results in the blood flowing from adds a new dimension to this research as well since it is able to visualize all the left to right atria will cause the blood pressure to increase in the right side cardiac structures under ~10 mmHg of pressure. -

Premature Obliteration of the Foramen Ovale by G
PREMATURE OBLITERATION OF THE FORAMEN OVALE BY G. AUSTIN GRESHAM From the Department of Pathology, University of Cambridge Received August 16, 1955 Obliteration of the foramen ovale occurring during intrauterine life is a rare condition. It throws some light on the mechanism of development of endocardial fibroelastosis and also upon the factors concerned in determining the size of the aorta and of the cardiac chambers. CASE HISTORY The patient was the second child of a mother (aet. 21) whose previous obstetric history was normal. Apart from two periods of rather rapid gain of weight for which no cause could be found, the pregnancy was uneventful. The child was eleven days postmature; labour was induced with an enema and lasted forty-five minutes. The infant cried lustily but was cyanosed: the heart was clinically normal. Abnormalities were present in all four limbs. The left radius and ulna were absent and rudimentary digits were present on the skin over the distal end of the limb. Terminal phalanges were absent in the fingers of the right hand, and four toes were present on each foot with a rudimentary fifth digit on the left foot. Cyanosis and dyspniea became more intense and the child died three hours after birth despite the use of oxygen. NECROPSY The body was that of a full-term male infant (weight 3430 g.). The limbs were abnormal as previously described. The lips were blue-black in colour. On the septal wall of the right atrium a hemispherical grey-white area (10x 12 x 4 mm. deep) with a central dimple filled in the usual site of the fossa ovalis (Fig. -
Cardiovascular System: Early Development
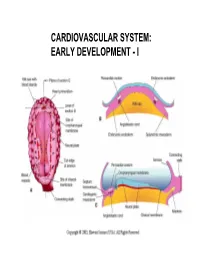
CARDIOVASCULAR SYSTEM: EARLY DEVELOPMENT - I CARDIOVASCULAR SYSTEM: EARLY DEVELOPMENT - II HEART AND ITS NEIGHBORHOOD- I HEART AND ITS NEIGHBORHOOD- II BLOOD VESSELS OF THE EMBRYO (at 26 days) FROM TUBE TO FOUR CHAMBERS EXTERNAL VIEW NORMAL : Loop to the RIGHT: Levocardia! ABNORMAL: Loop to the LEFT: Dextrocardia! FROM TUBE TO FOUR CHAMBERS INTERNAL VIEW FOUR CHAMBERS- ULTRASOUND VIEW @ 20 wks ATRIAL SEPTUM FORMATION- I ATRIAL SEPTUM FORMATION- II ATRIAL SEPTUM FORMATION- III ATRIAL SEPTUM FORMATION- IV ATRIAL SEPTUM FORMATION- V THE DEFINITIVE RIGHT ATRIUM **NOTE: In 25% of normal population, the foramen ovale remains ‘probe patent’. THE DEFINITIVE LEFT ATRIUM ATRIAL SEPTAL DEFECTS- “Fossa Ovalis” type ATRIAL SEPTAL DEFECTS- Unrelated to Foramen Ovale AORTIC ARCHES AND DERIVATIVES - I AORTIC ARCHES AND DERIVATIVES - II RECURRENT LARYNGEAL NERVES Right vs Left PATENT DUCTUS ARTERIOSUS Prostaglandin: Keeps the duct Patent Indomethacin: Closes the duct. RIGHT AORTIC ARCH: Mirror image branching ABERRANT RIGHT SUBCLAVIAN ARTERY: Occurs in 0.5% of people. Usually asymptomatic. DOUBLE AORTIC ARCH: “Vascular ring” Causes airway obstruction, stridor in infancy. COARCTATION OF THE AORTA THE CARDINAL VEINS AND THE VENAE CAVAE THE CARDINAL VEINS AND THE VENAE CAVAE SINUS VENOSUS AND THE CORONARY SINUS PERSISTENT LEFT SVC 0.3% of general population. 4 % of patients with Cong. Ht Dis. Usually drains to Coronary sinus. Usually asymptomatic. Enlarged coronary sinus is a clue. Left SVC to coronary sinus UMBILICAL AND VITELLINE VEINS- I: Liver, portal vein and ductus venosus. © 2005 Elsevier UMBILICAL AND VITELLINE VEINS- II: Liver, portal vein and ductus venosus. LYMPHATIC SYSTEM- I LYMPHATIC SYSTEM- II FETAL CIRCULATION POSTNATAL CIRCULATION. -

Unit 1 Lecture 2
Unit 1 Lecture 2 Unit 1 Lecture 2 THE HEART Anatomy of the Heart The heart rests on the diaphragm in a space called the mediastinum. It weighs @ 300 grams and is about as big as a clenched fist. The pointed end is called the apex and the opposite end is called the base but is really the top of the heart. The bulk of the heart tissue is made up of the left ventricle. A pericardium (a 3-layered bag) surrounds the heart and composed of fibrous pericardium (that provides protection to the heart, prevents overstretching, and anchors the heart in the mediastinum), a serous pericardium which is composed of two layers (the parietal layer or outer layer that is fused to the fibrous pericardium and the visceral layer or the inner layer that adheres to heart muscle). The visceral pericardium is also called the epicardium. Pericardial fluid is found in the pericardial cavity, which is located between the two layers, helps lubricate and reduces friction between membranes as the heart moves. The heart wall is composed of three layers: the epicardium (see visceral pericardium above), the myocardium which is cardiac muscle tissue and the bulk of mass in the heart, and endocardium or the innermost layer of the heart. This lining is continuous through all of the blood vessels except for the capillaries. The Heart Chambers and Valves Internally the heart is divided into four chambers. The two upper chambers are the right and left atria. Each has an appendage (auricle) whose function is to increase the volume of the atria. -

THE HEART Study Objectives
THE HEART Study Objectives: You are responsible to understand the basic structure of the four chambered heart, the position and structure of the valves, and the path of blood flow through the heart. Structure--The Outer Layer pericardium: a specialized name for the celomic sac that surrounds the heart. fibrous pericardium: the most outer, fibrous layer of the pericardium. serous pericardium: there are two surfaces (layers) to this tissue a) parietal layer: the outer layer of the serosa surrounding the heart. Lines the fibrous pericardium, and the combination of the two layers is also called the parietal pericardium. b) visceral layer: the inner layer of the serosa surrounding and adhering to the heart. Also called the visceral pericardium or epicardium. pericardial cavity: the potential space between the parietal and visceral layers of the serous pericardium. The cavity is filled with serous fluid Structure--The Heart: Blood is delivered to the right atrium from the coronary sinus, inferior vena cava and superior vena cava Blood travels to the right ventricle from the right atrium via the right atrioventricular valve Blood travels from the left atrium to the left ventricle via the left atrioventricular valve Blood leaves the heart via the pulmonary valve to the pulmonary trunk, is delivered to the lungs for oxygenation and returns via the pulmonary veins to the left atrium a) crista terminalis: separates the anterior rough-walled and posterior smooth-walled portions of the right atrium. b) right auricle: small, conical, muscular, pouch-like appendage. c) musculi pectinate (pectinate muscles): internal muscular ridges that fan out anteriorly from the crista terminalis. -

Download PDF File
Folia Morphol. Vol. 79, No. 4, pp. 736–741 DOI: 10.5603/FM.a2020.0009 O R I G I N A L A R T I C L E Copyright © 2020 Via Medica ISSN 0015–5659 journals.viamedica.pl Development of the interatrial wall during the ontogenesis of foetuses and children up to one year of age R. Kamiński1, A. Kosiński1, A. Kaczyńska1, M. Zajączkowski1, G. Piwko1, M. Gleinert-Rożek1, T. Gos2, K. Karnecki2 1Department of Clinical Anatomy, Medical University of Gdansk, Poland 2Department of Forensic Medicine, Medical University of Gdansk, Poland [Received: 29 October 2019; Accepted: 30 December 2019] Background: The foramen ovale, present in foetal interatrial septum, plays an important role during foetal life. During delivery, foramen ovale closes and be- comes fossa ovalis, starting the pulmonary circulation. The aim of our study was to describe the growth of the interatrial wall and changes in location of the foramen ovale, and fossa ovalis during the ontogenesis in the human hearts. Materials and methods: The study was performed on post-mortem material obtained from 92 human hearts from 22nd week of foetal life up to 1 year of age, fixed in a 4% formalin solution. Results: The interatrial wall size in the studied development period was greater in the horizontal than in the vertical dimension. During ontogenesis up to 1 year old, the anterior and inferior parts of the interatrial wall increased their shares considerably by 8% and 6%, respectively. The percentage participation of foramen ovale in the interatrial wall construction in the foetal period formed more than 50% of its size and fairly decreased reaching in infants about 39%. -

Study of Morphological Characteristics of Fossa Ovalis and Its Clinical Importance - a Cadaveric Study Dr
Scholars International Journal of Anatomy and Physiology Abbreviated Key Title: Sch Int J Anat Physiol ISSN 2616-8618 (Print) |ISSN 2617-345X (Online) Scholars Middle East Publishers, Dubai, United Arab Emirates Journal homepage: http://saudijournals.com/sijap/ Original Research Article Study of Morphological Characteristics of Fossa Ovalis and Its Clinical Importance - A Cadaveric Study Dr. Ashita Kaore1, Dr. Ashish Kamdi2*, Dr. N. Y. Kamdi3 1Assistant Professor, DepartmentofAnatomy, Government Medical College, Nagpur, Maharashtra, India 2Assistant Professor, Department of Anatomy, Government Medical College, Chhindwara, Madhya Pradesh, India 3Professor and Head, Department of Anatomy, Government Medical College, Nagpur Maharashtra, India *Corresponding author: Dr. Ashish Kamdi | Received: 02.03.2019 | Accepted: 05.03.2019 | Published: 30.03.2019 DOI:10.21276/sijap.2019.2.3.2 Abstract Transseptal access from right atrium to left atrium through the fossa ovalis using transseptal puncture and patent fossa ovalis repair are widely used cardiac techniques. These techniques require through knowledge of the cardiac anatomy especially of the fossa ovalis .Evaluation of the various morphological parameters of the fossa ovalis forms an important prerequisite before undertaking any surgical procedure in this region. This study was conducted on 60 cadaveric hearts from the department of Anatomy, Government medical college, Nagpur. The right atrium was opened and the fossa ovalis was studied for its shape, size, floor and the prominence and extent of the limbus fossa ovalis was noted. The fossa ovalis was observed for the presence of any patent foramen ovale or probe patency. In majority of the cases the shape of fossa ovalis was found to be oval 66.7% cases, the average craniocaudal diameter was found to be 15.03mm, and average anteroposterior diameter was found to be 14.44mm. -

Chapter Twenty
Chapter 22 Outline • Overview of the Cardiovascular System • Anatomy of the Heart • Coronary Circulation • How the Heart Beats: Electrical Properties of Cardiac Tissue • Innervation of the Heart • Tying It All Together: The Cardiac Cycle • Aging and the Heart • Development of the Heart Overview of the Cardiovascular System • The heart propels blood to and from most body tissues via two basic types of blood vessels called ______ and ______. • Arteries are defined as blood vessels that carry blood away from the heart. • Veins are defined as blood vessels that carry blood back to the heart. • The arteries and veins entering and leaving the heart are called ______ vessels. General Characteristics and Functions of the Heart • Blood flow through the heart is ______ because of four valves within the heart. • The heart is functionally two side-by-side pumps that work at the same rate and pump the same volume of blood. – One pump directs blood to the lungs. – One pump directs blood to most body tissues. General Characteristics and Functions of the Heart • The heart generates ______ pressure through alternate cycles of the heart wall’s contraction and relaxation. • Blood pressure is the force of the blood pushing against the inside walls of blood vessels. • A minimum blood pressure is essential to circulate blood throughout the body. Pulmonary and Systemic Circulations The cardiovascular system consists of two circulations: 1. ______—right side of the heart and the pulmonary arteries and veins; conveys blood to the lungs and back to the left side of the heart 2. ______—left side of the heart and arteries and veins; conveys blood to most body tissues and back to the right side of the heart Cardiovascular System Figure 22.1 Position of the Heart • Slightly left of midline deep to the sternum in a compartment of the thorax known as the mediastinum Figure 22.2 Position of the Heart • During development, the heart rotates such that the right side or right border (primarily formed by the right atrium and ventricle) is located more anteriorly. -

Development of Heart Notes
Formation of heart tube: 3rd week Heart beat: 22nd –23rd day (beginning of fourth week) USG detection of heart beat: 7th week Foetal ECG: 11th week Endocardium from original heart tube Myocardium from surrounding mesoderm & epicardium (myoepicardial mantle) (visceral pericardium) Lining of pericardium epithelium of pericardial cavity Transverse sinus formed by disappearance of dorsal mesocardium (Present between arterial and venous ends of the heart tube) FATE Of SINUS VENOSUS Left horn of sinus venosus, along with medial part of common cardinal vein forms coronary sinus Lateral part of common cardinal vein forms oblique vein of left atrium Left venous valve merges with septum secundum. Right venous valve is divided in three parts by appearance of two transverse muscular bands, called limbic bands. i) The part above superior limbic band forms crista terminalis ii) The part between the two bands forms valve of inferior vena cava iii) The part below the inferior limbic band forms valve of coronary sinus INTERATRIAL SEPTUM i) Upper, thicker part is formed by septum secundum ii) Lower, thin part (floor of fossa ovalis) is formed by septum primum iii) Sharp margin of fossa ovalis is formed by lower, curved margin of septum secundum DEVELOPMENT OF RIGHT ATRIUM It develops from 1. Right half of primitive atrial chamber (rough part); 2. Absorption of right horn of sinus venosus (smooth part) and 3. Right atrioventricular canal. DEVELOPMENT OF LEFT ATRIUM It develops from 1. Left half of primitive atrial chamber (rough part – confined to the auricle); 2. Absorption of pulmonary veins (smooth part) and 3. Left atrioventricular canal. -

22. Heart.Pdf
CARDIOVASCULAR SYSTEM OUTLINE 22.1 Overview of the Cardiovascular System 657 22.1a Pulmonary and Systemic Circulations 657 22.1b Position of the Heart 658 22 22.1c Characteristics of the Pericardium 659 22.2 Anatomy of the Heart 660 22.2a Heart Wall Structure 660 22.2b External Heart Anatomy 660 Heart 22.2c Internal Heart Anatomy: Chambers and Valves 660 22.3 Coronary Circulation 666 22.4 How the Heart Beats: Electrical Properties of Cardiac Tissue 668 22.4a Characteristics of Cardiac Muscle Tissue 668 22.4b Contraction of Heart Muscle 669 22.4c The Heart’s Conducting System 670 22.5 Innervation of the Heart 672 22.6 Tying It All Together: The Cardiac Cycle 673 22.6a Steps in the Cardiac Cycle 673 22.6b Summary of Blood Flow During the Cardiac Cycle 673 22.7 Aging and the Heart 677 22.8 Development of the Heart 677 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch22_656-682.indd 656 2/14/11 4:29 PM Chapter Twenty-Two Heart 657 n chapter 21, we discovered the importance of blood and the which carry blood back to the heart. The differences between I myriad of substances it carries. To maintain homeostasis, blood these types of vessels are discussed in chapter 23. Most arteries must circulate continuously throughout the body. The continual carry blood high in oxygen (except for the pulmonary arteries, pumping action of the heart is essential for maintaining blood as explained later), while most veins carry blood low in oxygen circulation. If the heart fails to pump adequate volumes of blood, (except for the pulmonary veins).