Clearwater River Basin Spring Chinook Salmon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Environmental Impact Statement Nez Perce Tribal Hatchery Program
Final Environmental Impact Statement Nez Perce Tribal Hatchery Program Bonneville Power Administration U.S. Department of Energy Bureau of Indian Affairs U.S. Department of the Interior Nez Perce Tribe July 1997 Final Environmental Impact Statement Responsible Agencies: U.S. Department of Energy, Bonneville Power Administration (BPA); U.S. Department of the Interior, Bureau of Indian Affairs (BIA); Nez Perce Tribe (NPT). Title of Proposed Action: Nez Perce Tribal Hatchery Program. States Involved: Idaho. Abstract: Bonneville Power Administration, the Bureau of Indian Affairs, and the Nez Perce Tribe propose a supplementation program to restore chinook salmon to the Clearwater River Subbasin in Idaho. The Clearwater River is a tributary to the Snake River, which empties into the Columbia River. The Final EIS includes a new alternative suggested by commentors to the Draft EIS. In the Proposed Action, the Nez Perce Tribe would build and operate two central incubation and rearing hatcheries and six satellite facilities. Spring and fall chinook salmon would be reared and acclimated to different areas in the Subbasin and released at the hatchery and satellite sites or in other watercourses throughout the Subbasin. The supplementation program differs from other hatchery programs because the fish would be released at different sizes and would return to reproduce naturally in the areas where they are released. The Use of Existing Facilities Alternative proposes using existing production hatcheries and the proposed satellite facilities to meet the need. Facilities at Dworshak National Fish Hatchery, Kooskia National Fish Hatchery, and Hagerman National Fish Hatchery would be used as central incubation and rearing facilities. -

Draft Clearwater Assessment: 8. Fishery Resources
8 Fishery Resources 8.1 Fish Status Currently more than 30 species of fish inhabit the Clearwater subbasin, including 19 native species, two of which have been reintroduced (Table 43). Salmonids and cyprinids are most numerous, representing 10 and 6 species, respectively. Exotic species within the subbasin are generally introduced sport or forage species, and include primarily centrarchids, ictalurids, and salmonids. Five fish species have been chosen as aquatic focal species in this assessment: chinook salmon (Oncorhynchus tshawytscha), steelhead trout (Oncorhynchus mykiss subspecies), westslope cutthroat trout (Oncorhynchus clarki lewisi), bull trout (Salvelinus confluentus) and brook trout (Salvelinus fontinalis). Aquatic focal species may serve as indicators of larger communities, and are listed by federal and/or state agencies as species of concern or, in the case of brook trout, have the potential to negatively impact other selected species. In addition, aquatic focal species had adequate data available for species status, distribution, and habitat use to aid future decision making. Information is also provided for additional species of interest for which only limited data exists, redband trout (Oncorhynchus mykiss subspecies), Pacific lamprey (Lampetra tridentata) and coho salmon (Oncorhynchus kisutch). Although species status is discussed, data limitations for these species prohibits substantial consideration of limiting factors and distribution or condition of existing habitat areas. The resident fishery in Dworshak Reservoir is also considered a substantial fishery resource in the Clearwater subbasin. The Dworshak Reservoir fishery involves multiple species, and is addressed as a single fishery rather than as a large number of individual species. Distribution and status information was compiled for the five aquatic focal species using 23 data sources. -
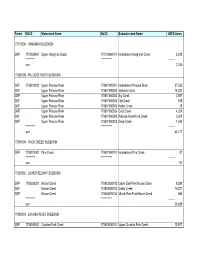
Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres
Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres 17010306 - HANGMAN SUBBASIN CNF 1701030601 Upper Hangman Creek 170103060101 Headwaters Hangman Creek 2,245 ********** ************ --------- sum 2,245 17060108 - PALOUSE RIVER SUBBASIN CNF 1706010803 Upper Palouse River 170601080301 Headwaters Palouse River 27,352 CNF Upper Palouse River 170601080302 Meadow Creek 14,237 CNF Upper Palouse River 170601080303 Big Creek 2,857 CNF Upper Palouse River 170601080304 Flat Creek 839 CNF Upper Palouse River 170601080305 Hatter Creek 16 CNF Upper Palouse River 170601080306 Gold Creek 4,224 CNF Upper Palouse River 170601080308 Palouse River/Rock Creek 3,300 CNF Upper Palouse River 170601080309 Deep Creek 1,346 ********** ************ --------- sum 54,171 17060109 - ROCK CREEK SUBBASIN CNF 1706010901 Pine Creek 170601090101 Headwaters Pine Creek 87 ********** ************ --------- sum 87 17060302 - LOWER SELWAY SUBBASIN CNF 1706030201 Moose Creek 170603020102 Upper East Fork Moose Creek 8,290 CNF Moose Creek 170603020103 Cedar Creek 16,271 CNF Moose Creek 170603020104 Middle East Fork Moose Creek 686 ********** ************ --------- sum 25,639 17060303 - LOCHSA RIVER SUBBASIN CNF 1706030301 Crooked Fork Creek 170603030101 Upper Crooked Fork Creek 18,907 Forest HUC5 Watershed Name HUC6 Subwatershed Name USFS Acres CNF Crooked Fork Creek 170603030102 Boulder Creek 15,627 CNF Crooked Fork Creek 170603030103 Lower Crooked Fork Creek 11,766 CNF Crooked Fork Creek 170603030104 Upper Brushy Fork Creek 5,142 CNF Crooked Fork Creek 170603030105 Spruce -

Clearwater Rapid Watershed Assessment
Clearwater - 17060306 Idaho 8 Digit Hydrologic Unit Profile December 2006 Tensed Farmington Clearwater - 17060306 8-Digit Hydrologic Unit LATAHLATAH Location Map Potlatch Bovill WHITMAN WHITMAN Deary (Washington) (Washington) Elk River Troy Moscow CLEARWATERCLEARWATER Kendrick Juliaetta Genesee Pierce Clearwater Peck Orofino Clarkston 17060306 Lapwai Culdesac Weippe Lewiston Asotin Reubens LEWISLEWIS Kamiah Craigmont Winchester Nezperce Kooskia Ferdinand NEZNEZ Stites PERCEPERCE Cottonwood !COEUR d ALENE MOSCOW! IDAHOIDAHO Grangeville LEWISTON! !REXBURG CALDWELL! !BOISE NAMPA! !IDAHO FALLS : BLACKFOOT ! !POCATELLO 0 5 10 15 20 25 TWIN! FALLS Miles The United States Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, and marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326W, Whitten Building, 14 th and Independence Avenue, SW, Washington DC 20250-9410 or call (202) 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. 1 Clearwater - 17060306 Idaho 8 Digit Hydrologic Unit Profile December 2006 Introduction The Clearwater 8-Digit Hydrologic Unit Code (HUC) subbasin is 1,503,992 acres. Clearwater and Nez Perce counties each account for approximately 24 percent of the subbasin. Twenty two percent of the subbasin is in Latah County, 18 percent in Lewis County, 13 percent in Idaho County and less than 0.5% in Whitman County, Washington. -

Fishing and Boating Access Guide 2016
CLEARWATER REGION IDAHO FISHING & BOATING ACCESS GUIDE 10 Onaway 6 P! 3 SHOSHONE RQ P! Princeton RQ6 RQ Potlatch P P WASHINGTON Harvard ¤£ Palouse River Panhandle 95 Moose Creek Reservoir In-State X Location iver P! Bovill ClearwaterFork Clearwater R P Viola rth RQ9 No P Vassar Spring Valley Reservoir RQ3 Salmon Deary P! P Upper Snake er RQ8 S pX Helmer iv r in g R V h P! Southwest Magic a Elk River l tc South le tla LATAH y Po Valley R 8 d RQ X -east !P Moscow TroyP! RQ8 P Elk Creek Reservoir Joel RQ3 RQ99 CLEARWATER Deer Creek Reservoir ¤£ 95 P! Kendrick Headquarters P P X Cameron P Southwick Dworshak McKays Eddy - Myrtle P! Reservoir Juliaetta Clearwater Hatchery P! Ahsahka Bridge Genesee RQ3 P Cavendish Campbell Pond Cherry Lane Lenore North Fork Clearwater River X P X wate Jaype ar r R P Lenore P Grangemont Myrtle Cle iv Upper Hog Island P er X X 12 XXPX RQ X XXX P Riverside rofino Cre ek RQ11 X Ahsahka O P! Lower Hog Island P! Peck P! Pierce ¤£12 McGill Spur Orofino X XX P Spalding Gibbs Eddy RQ128 ¤£ Ahsahka !P 12 RQ7 Zans Lewiston Beardy Gulch L in P! d Lapwai X s a y C rk R d Powers Rd Sweetwater Culdesac Greer XP X P P P! Jacques 11 P! Weippe ek RQ ig C re Greer X ¤£95 B anyon C X N E Z P E R C E C le a d ! r Deyo Reservoir P Five-Mile Creek w k R e Reubens a a e t h r e a Mann Lake C r P i R Woodland W a LEWIS iv w e Lolo p r C reek S n a RQ62 a L k e Winchester Lake Longcamp X R i v e P! r Winchester P! 64 Waha Lake X Craigmont RQ Nezperce P! P Glenwood P Waha P! Kamiah X ¤£ wyer 95 RQ162 La C reek IDAHO X X IDAHO FISHING -

Mylie Lawyer and Her Collection
National Park Service Nez Perce NHP U.S. Department of the Interior Big Hole National Battlefield Nez Perce National Historical Park Mylie Lawyer And Her Collection Mylie Lawyer has had an interest in her family Hair, one of the Nez Perce leaders who welcomed history since she was a child growing up in Lapwai, Lewis and Clark in 1805. Mylie's collection stands as Idaho in the 1920s. Her perseverance has resulted in testimony to her determination to preserve her an extensive collection of stories and artifacts related family's heritage. to her family that includes Chief Lawyer and Twisted Mylie Lawyer Mylie Lawyer was born on the Pine Ridge stones from an area where her family and Born: March 24, 1912 Indian Agency, South Dakota to Corbett ancestors had camped. and Lillian Allman Lawyer. Corbett worked for the Bureau of Indian Affairs She also has fond memories of sitting with (BIA) and Lillian was a teacher. her dad, Corbett and other Nez Perce men as they spoke in the Nez Perce language, After Mylie was born, the family returned telling stories, plucking whiskers, fishing to Lapwai, Idaho where Mylie grew up. and remembering the old days. Mylie did After graduating from Lapwai High School not speak Nez Perce so after these ses- in 1929, she attended Oregon State and sions, she would always ask her dad to Willamette University where she earned a recount the things they had talked about. degree in Home Economy. Even in her college days as she studied home economics she wished she had stud- In the 1930s she worked at the Warm ied anthropology or history instead. -

Values of the Middle Fork Clearwater Adn Lochsa River Corridor
Values of the Middle Fork Clearwater and Lochsa River Corridor Potentially Affected by Certain Over-Legal Truck Traffic US Highway 12 US Forest Service Northern Region Missoula, Montana March, 2015 ______________________________________________________________________________ BACKGROUND ............................................................................................................................ 1 PURPOSE AND LIMITATIONS................................................................................................... 2 HISTORICAL CONTEXT ............................................................................................................. 2 US HIGHWAY 12 – IDAHO SEGMENT ..................................................................................... 3 Agency Roles and Responsibilities ............................................................................................ 4 Idaho Transportation Department (ITD) ................................................................................. 4 Federal Highway Administration (FHWA) ............................................................................. 4 Forest Service .......................................................................................................................... 5 Over-Legal Sized Load Traffic .................................................................................................. 5 NATIONAL DESIGNATIONS WITHIN THE CORRIDOR ....................................................... 6 Wild and Scenic River: Middle Fork -

Movement Patterns of Adult Steelhead and Anglers in the Mainstem Clearwater River, Idaho
Movement Patterns of Adult Steelhead and Anglers in the Mainstem Clearwater River, Idaho Stacey F. Feeken Idaho Cooperative Fish and Wildlife Research Unit, Department of Fish and Wildlife Sciences, University of Idaho Michael C. Quist U.S. Geological Survey, Idaho Cooperative Fish and Wildlife Unit, Department of Fish and Wildlife Sciences, University of Idaho Brett J. Bowersox and Marika E. Dobos Idaho Department of Fish and Game Clearwater River Columbia River Snake River 0 100 km Potlatch River Five wild populations NF Clearwater River • Lower Clearwater River • Lolo Creek Clearwater River • SF Clearwater River • Lochsa River MF Clearwater River Lochsa River • Selway River One hatchery stock Selway River SF Clearwater River Trophy steelhead fishery July 1st • Catch and release Entire river (to Clear Creek) August 1st • Catch and keep Mouth to Memorial Bridge • Catch and release Entire river (to Clear Creek) October 15th • Catch and keep Entire river (to Clear Creek) May 1st • Closed to fishing How can we better understand the steelhead fishery to benefit both steelhead and anglers? Describe steelhead distribution and movement • Wild and hatchery steelhead • Timing into the Clearwater River • Fate Describe angler distribution Methods Sort-By-Code Clearwater River Columbia River GO LM LG DW IH MC JD TD BO HC Snake River 0 100 km Lower Granite Dam Potlatch River NF Clearwater River Tracking 1 2 3 4 5 Clearwater River 6 MF Clearwater River 7 Lochsa River 8 Selway River SF Clearwater River 0 40 km Results Lower Clearwater -
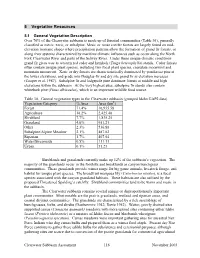
Draft Clearwater Assessment: 5. Vegetative Resources
5 Vegetative Resources 5.1 General Vegetation Description Over 70% of the Clearwater subbasin is made up of forested communities (Table 30), generally classified as mesic, xeric, or subalpine. Mesic or moist conifer forests are largely found on mid- elevation montane slopes where precipitation patterns allow the formation of grand fir forests, or along river systems characterized by maritime climatic influences such as occur along the North Fork Clearwater River and parts of the Selway River. Under these unique climatic conditions grand fir gives way to western red cedar and hemlock (Tsuga heterophylla) stands. Cedar forests often contain unique plant species, including two focal plant species, crenulate moonwort and mountain moonwort. Xeric or dry forests are characteristically dominated by ponderosa pine at the lower elevations, and grade into Douglas-fir and dry site grand fir as elevation increases (Cooper et al. 1987). Subalpine fir and lodgepole pine dominate forests at middle and high elevations within the subbasin. At the very highest sites, subalpine fir stands also contain whitebark pine (Pinus albicaulus), which is an important wildlife food source. Table 30. General vegetation types in the Clearwater subbasin (grouped Idaho GAP2 data) Vegetation Category % Area Area (km2) Forest 71.4% 16,955.58 Agriculture 10.2% 2,425.48 Shrubland 7.7% 1,835.25 Grassland 4.0% 951.21 Other 2.3% 536.88 Subalpine/Alpine Meadow 2.1% 487.02 Riparian 1.7% 407.04 Water/Streamside 0.5% 111.11 Urban 0.1% 31.23 Shrublands and grasslands currently make up 12% of the subbasin’s vegetation. The majority of the grasslands occur in the foothills and breaklands as canyon bunchgrass communities. -

Ez Perce ~Clearwater Forests N Lochsa Ranger Station, After 1935 Fire
ezez PercePerce ~~ClearwaterClearwater National N Forests VISITOR GUIDE Where Wild Country Meets Wild Rivers Split Creek Bridge on the Lochsa River (Morai Helfen) he Nez Perce-Clearwater National Forests What’s Inside Tin north central Idaho begin in the jagged peaks of the Bitterroot Mountains and flow Get to Know Us........................... 2 to the deep canyons of the Salmon, Selway Scenic Byways .......................... 4 and Lochsa Rivers. Broad coniferous forests Historic Roads & Trails.............. 5 stretch from the rolling hills of the Palouse Wild & Scenic Rivers ................ 6 Wilderness .................................7 Plateau to the Camas Prairie. Salmon River ............................. 8 Map ........................................... 10 Nez Perce drummer The forests are home to an extravagant Activities ...................................12 array of wildlife, from the strutting grouse Winter Recreation ...................... 16 More Activities ................................ 17 to the stately bighorn sheep; golden eagles Wildlife ......................................18 soar above; river otters glide through waters Know Before You Go................. 19 below. Blue ribbon fisheries lure anglers, Contact Information & Fees.... 20 (Cindy Schacher) Camas Fun Forest Facts while backcountry pack trails beckon hunters. Visitors enjoy activities year-round— motorized and non-motorized, on rivers and mountaintops. For the Acres: 4 million Longest Trail: The Nez Perce more adventurous, the untrammeled land of Wilderness provides National Historic Trail stretches recreation opportunities while preserving natural landscapes into 1,170 miles crossing Oregon, perpetuity. Idaho, Montana, and Wyoming Deepest Gorge: The Snake River/ Hells Canyon and the Salmon As the traditional homeland of the Nez Perce Tribe, the forests River Canyon are deeper than the value their history, culture, and knowledge of the land, while Grand Canyon conserving the resources of this special place for all people. -

Lochsa River
LOCHSA RIVER The Lochsa River is one of the world’s greatest whitewater trips and was one of the original six rivers designated “Wild and Scenic” by the United States Congress in 1968 as part of the federal Wild and Scenic Rivers Act. The river is surrounded by National Forest lands, the Selway-Bitterroot Wilderness Area and flows through a dramatic canyon of thick cedar, fir and pine forests. ROW offers trips from one to three days and all of our trips are based out of River Dance Lodge. At the Lodge you may stay in handcrafted log cabins, glamping tents or camp in our camping area. Dining is available at the Syringa Café where you’ll find great food, a delicious wine list, microbrews on tap and a friendly, hospitable staff. The Lodge is an ideal base for other exploration of the area including the Lewis and Clark Trail, Selway Falls, and an extensive trail system in the nearby National Forest lands. When to go: Our Lochsa season starts in early May and goes until the water gets too low for rafting, which can be anytime from the early to mid-July. When choosing your trip, keep in mind that the river is more demanding in mid-May and the beginning of June when the water is high. During late June and July the water lowers, exposing more rocks and making it a more technical run. Since the river is not dammed, water flows depend strictly on snow pack. During higher water, we use only 16' paddle-assisted oar-rafts. -

Nez Perce Clearwater River Subbasin Climate Change Adaptation Plan
1 Clearwater River Subbasin Climate Change Adaptation Plan 2011 Clearwater River Subbasin Climate Change Adaptation Plan Nez Perce Tribe Water Resources Division Model Forestry Policy Program Cumberland River Compact 12/30/2011 1 2 Clearwater River Subbasin Climate Change Adaptation Plan Clearwater River Subbasin Climate Change Adaptation Plan Published December 2011 Primary Authors: Ken Clark Jenifer Harris Action Plan Contributors: Nez Perce Tribe Water Resources Division University of Idaho Nick and Marci Gerhardt Columbia River Intertribal Fish Commission USDA Forest Service-Nez Perce and Clearwater National Forests Senator Risch’s Office Senator Crapo’s Office Editors: Toby Thaler, JD Gwen Griffith, DVM, MS For more information, please contact: Ken Clark Water Quality Coordinator Nez Perce Tribe [email protected] 208-843-7368, ext. 3903 Big Canyon Creek, Tributary to the Clearwater River 2 3 Clearwater River Subbasin Climate Change Adaptation Plan Contents Foreword ......................................................................................................................................... 8 Executive Summary ...................................................................................................................... 10 Overview of Climate Change and Resource Management in the Clearwater River Subbasin ..... 13 Clearwater River Subbasin Overview ....................................................................................... 14 Resource Governance and Planning ........................................................................................