Evaluation of Water Quality of Thuthapuzha Sub-Basin Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Report of the Malabar Puffer, Carinotetraodon Travancoricus
Journal on New Biological Reports 1(2): 42-46 (2012) ISSN 2319 – 1104 (Online) The first report of the Malabar puffer, Carinotetraodon travancoricus (Hora & Nair, 1941) from the Neyyar wildlife sanctuary with a note on its feeding habit and length-weight relationship G. Prasad*, K. Sabu and P.V. Prathibhakumari Laboratory of Conservation Biology, Department of Zoology, University of Kerala, Kariavattom, Thiruvananthapuram 695 581, Kerala, India (Received on: 20 October, 2012; accepted on: 2 November, 2012) ABSTRACT Carinotetraodon travancoricus, the Malabar puffer fish has been collected and reported for first time from the Kallar stream, Neyyar Wildlife Sanctuary of southern part of Kerala. The food and feeding habit and length-weight relationship of the fish also has been studied and presented. Key words : Carinotetraodon travancoricus, Neyyar Wildlife Sanctuary, Kallar stream, length- weight relationship INTRODUCTION The Western Ghats of India along with Sri Lanka is Carinotetraodon travancoricus commonly considered as one of the biodiversity hotspots of the known as Malabar puffer fish inhabits in freshwater world (Mittermeier et al. 1998; Myers et al. 2000). and estuaries which is endemic to Kerala and This mountain range extends along the west coast of Karnataka (Talwar & Jhingran 1991; Jayaram 1999; India and is crisscrossed with many streams, which Remadevi 2000). Carinotetraodon travancoricus was form the headwaters of several major rivers draining first described from Pamba River by Hora & Nair water to the plains of peninsular India. The Ghats is a (1941). This fish is present in 13 rivers of Kerala critical ecosystem due to its high human population including Chalakudy, Pamba, Periyar, Kabani, pressure (Cincotta et al. -

KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with Financial Assistance from the World Bank
KERALA SOLID WASTE MANAGEMENT Public Disclosure Authorized PROJECT (KSWMP) INTRODUCTION AND STRATEGIC ENVIROMENTAL ASSESSMENT OF WASTE Public Disclosure Authorized MANAGEMENT SECTOR IN KERALA VOLUME I JUNE 2020 Public Disclosure Authorized Prepared by SUCHITWA MISSION Public Disclosure Authorized GOVERNMENT OF KERALA Contents 1 This is the STRATEGIC ENVIRONMENTAL ASSESSMENT OF WASTE MANAGEMENT SECTOR IN KERALA AND ENVIRONMENTAL AND SOCIAL MANAGEMENT FRAMEWORK for the KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with financial assistance from the World Bank. This is hereby disclosed for comments/suggestions of the public/stakeholders. Send your comments/suggestions to SUCHITWA MISSION, Swaraj Bhavan, Base Floor (-1), Nanthancodu, Kowdiar, Thiruvananthapuram-695003, Kerala, India or email: [email protected] Contents 2 Table of Contents CHAPTER 1. INTRODUCTION TO THE PROJECT .................................................. 1 1.1 Program Description ................................................................................. 1 1.1.1 Proposed Project Components ..................................................................... 1 1.1.2 Environmental Characteristics of the Project Location............................... 2 1.2 Need for an Environmental Management Framework ........................... 3 1.3 Overview of the Environmental Assessment and Framework ............. 3 1.3.1 Purpose of the SEA and ESMF ...................................................................... 3 1.3.2 The ESMF process ........................................................................................ -

Rain 11 08 2019.Xlsx
Rainfall in 'mm' on 11.08.2019 District River Basin Station Name 11-08-2019 Alappuzha Achencovil Kollakadavu 55.2 Alappuzha Manimala Ambalapuzha 99.3 Alappuzha Muvattupuzha Arookutty 114.4 Alappuzha Muvattupuzha Cherthala 108 Cannanore Anjarakandy Cheruvanchery 96 Cannanore Anjarakandy F.c.s. Pazhassi 93 Cannanore Anjarakandy Kottiyoor 176 Cannanore Anjarakandy Kannavam 72 Cannanore Karaingode Pulingome 167.4 Cannanore Kuppam Alakkode 148.6 Cannanore Peruvamba Kaithaprem 116.2 Cannanore Peruvamba Olayampadi 144.6 Cannanore Ramapuram Cheruthazham 70.2 Cannanore Anjarakandy Maloor 104 Cannanore Valapattanam Mangattuparamba 58.6 Cannanore Anjarakandy Nedumpoil 77.2 Cannanore Valapattanam Palappuzha 80 Cannanore Valapattanam Payyavoor 140 Cannanore Kuppam Alakkode 148.6 Cannanore Valapattanam Thillenkeri 121 Ernakulam Muvattupuzha Piravam 87.2 Ernakulam Periyar Aluva 112.5 Ernakulam Periyar Boothathankettu 79.6 Ernakulam Periyar Keerampara 63.2 Ernakulam Periyar Neriyamangalam 69.8 Idukki Manimala Boyce estate 47 Idukki Muvattupuzha Vannapuram 54.3 Idukki Pambar Marayoor 5.6 Idukki Periyar Chinnar 37 Idukki Periyar FCS Painavu 32.4 Idukki Periyar Kumali 27 Idukki Periyar Nedumkandam 23.8 Idukki Periyar Vandanmedu 34.8 Kasaragod Chandragiri Vidhyanagar 161.8 Kasaragod Chandragiri Kalliyot 142.3 Kasaragod Chandragiri Padiyathadukka 126.4 Kasaragod Karaingode Kakkadavue(cheemeni)fcs 141.8 Kasaragod Manjeswar Manjeswaram 74 Kasaragod Morgal Madhur 145.2 Kasaragod Nileswar Erikkulam 127.4 Kasaragod Shiriya Paika 137 Kasaragod Uppala Uppala 90.5 -

A CONCISE REPORT on BIODIVERSITY LOSS DUE to 2018 FLOOD in KERALA (Impact Assessment Conducted by Kerala State Biodiversity Board)
1 A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) Editors Dr. S.C. Joshi IFS (Rtd.), Dr. V. Balakrishnan, Dr. N. Preetha Editorial Board Dr. K. Satheeshkumar Sri. K.V. Govindan Dr. K.T. Chandramohanan Dr. T.S. Swapna Sri. A.K. Dharni IFS © Kerala State Biodiversity Board 2020 All rights reserved. No part of this book may be reproduced, stored in a retrieval system, tramsmitted in any form or by any means graphics, electronic, mechanical or otherwise, without the prior writted permission of the publisher. Published By Member Secretary Kerala State Biodiversity Board ISBN: 978-81-934231-3-4 Design and Layout Dr. Baijulal B A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) EdItorS Dr. S.C. Joshi IFS (Rtd.) Dr. V. Balakrishnan Dr. N. Preetha Kerala State Biodiversity Board No.30 (3)/Press/CMO/2020. 06th January, 2020. MESSAGE The Kerala State Biodiversity Board in association with the Biodiversity Management Committees - which exist in all Panchayats, Municipalities and Corporations in the State - had conducted a rapid Impact Assessment of floods and landslides on the State’s biodiversity, following the natural disaster of 2018. This assessment has laid the foundation for a recovery and ecosystem based rejuvenation process at the local level. Subsequently, as a follow up, Universities and R&D institutions have conducted 28 studies on areas requiring attention, with an emphasis on riverine rejuvenation. I am happy to note that a compilation of the key outcomes are being published. -
List of Lacs with Local Body Segments (PDF
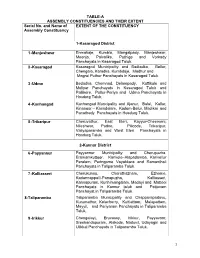
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -

Environmental Analysis Report for Kerala
E-355 VOL. 2 REVISED Environmental Analysis Report Public Disclosure Authorized for Kerala Rural Water Supply and Sanitation (KRWSS) Project Public Disclosure Authorized 30 th May, 2000 Public Disclosure Authorized Prepared for The World Bank, Washington D.C. and Kerala Rural Water Supply and Sanitation Agency Prepared by Public Disclosure Authorized Dr. R. Paramasivam (Consultant) CONTENTS CHAPTER TITLE PAGE Executive Summary 1. Introduction 1.1. Background 1.1 1.2. Environmental Analysis Study 1.2 1.3. Methodology 1.2 1.4. Organisation of the Report 1.4 2. Policy, Legal and Administrative Framework for Environmental Analysis 2.1. EA Requirements for Project Proposed for IDA Funding 2.1 2.2. Ministry of Environment & Forests, GOI Requirements 2.1 2.3. Kerala State Water Policy 2.3 2.4. Water Quality Monitoring 2.6 2.5. State Ground Water legislation 2.11 2.6. Statutory Requirements of State Pollution Control Board 2.12 2.7. Coastal Zone Management (CZM) Plan of Kerala 2.12 3. Project Description 3.1. Project Development Objective 3.1 3.2. Project Scope and Area 3.1 3.3. Project Components 3.2 3.4. Project Cost and Financing Plan 3.4 3.5. Institutional Arrangement 3. 6 X 3.6. Project Implementation Schedule and Scheme Cycle 3.9 3.7. Expected Benefits of the Project 3.9 4. Baseline Environmental Status 4.1. Physical Environment 4.1 Location & Physiography Geology Rainfall Climate 4.2. Water Environment 4.5 Surface Water Resources Surface Water Quality Salinity Intrnsion Hydrogeology Groundwater Potential and Utilisation in Kerala Groundwater -

Ground Water Information Booklet of Alappuzha District
TECHNICAL REPORTS: SERIES ‘D’ CONSERVE WATER – SAVE LIFE भारत सरकार GOVERNMENT OF INDIA जल संसाधन मंत्रालय MINISTRY OF WATER RESOURCES कᴂ द्रीय भजू ल बो셍 ड CENTRAL GROUND WATER BOARD केरल क्षेत्र KERALA REGION भूजल सूचना पुस्तिका, मलꥍपुरम स्ज쥍ला, केरल रा煍य GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA STATE तत셁वनंतपुरम Thiruvananthapuram December 2013 GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA जी श्रीनाथ सहायक भूजल ववज्ञ G. Sreenath Asst Hydrogeologist KERALA REGION BHUJAL BHAVAN KEDARAM, KESAVADASAPURAM NH-IV, FARIDABAD THIRUVANANTHAPURAM – 695 004 HARYANA- 121 001 TEL: 0471-2442175 TEL: 0129-12419075 FAX: 0471-2442191 FAX: 0129-2142524 GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA TABLE OF CONTENTS DISTRICT AT A GLANCE 1.0 INTRODUCTION ..................................................................................................... 1 2.0 CLIMATE AND RAINFALL ................................................................................... 3 3.0 GEOMORPHOLOGY AND SOIL TYPES .............................................................. 4 4.0 GROUNDWATER SCENARIO ............................................................................... 5 5.0 GROUNDWATER MANAGEMENT STRATEGY .............................................. 11 6.0 GROUNDWATER RELATED ISaSUES AND PROBLEMS ............................... 14 7.0 AWARENESS AND TRAINING ACTIVITY ....................................................... 14 -

Biodiversity Status.Qxp
163 BIODIVERSITY STATUS OF FISHES INHABITING RIVERS OF KERALA (S. INDIA) WITH SPECIAL REFERENCE TO ENDEMISM, THREATS AND CONSERVATION MEASURES Kurup B.M. Radhakrishnan K.V. Manojkumar T.G. School of Industrial Fisheries, Cochin University of Science & Technology, Cochin 682 016, India E-mail: [email protected] ABSTRACT The identification of 175 freshwater fish- es from 41 west flowing and 3 east flowing river systems of Kerala were confirmed. These can be grouped under 106 ornamental and 67 food fish- es. The biodiversity status of these fishes was assessed according to IUCN criteria. The results showed that populations of the majority of fish species showed drastic reduction over the past five decades. Thirty-three fish species were found to be endemic to the rivers of Kerala. The distributions of the species were found to vary within and between the river systems and some of the species exhibited a high degree of habitat specificity. The diversity and abundance of the species generally showed an inverse relationship with altitude. The serious threats faced by the freshwater fishes of Kerala are mostly in the form of human interventions and habitat alter- ations and conservation plans for the protection and preservation of the unique and rare fish bio- diversity of Kerala are also highlighted. 164 Biodiversity status of fishes inhabiting rivers of Kerala (S.India) INTRODUCTION river. Habitat diversity was given foremost importance during selection of locations within the river system. Kerala is a land of rivers which harbour a rich The sites for habitat inventory were selected based on and diversified fish fauna characterized by many rare channel pattern, channel confinement, gradient and and endemic fish species. -

Report of Rapid Impact Assessment of Flood/ Landslides on Biodiversity Focus on Community Perspectives of the Affect on Biodiversity and Ecosystems
IMPACT OF FLOOD/ LANDSLIDES ON BIODIVERSITY COMMUNITY PERSPECTIVES AUGUST 2018 KERALA state BIODIVERSITY board 1 IMPACT OF FLOOD/LANDSLIDES ON BIODIVERSITY - COMMUnity Perspectives August 2018 Editor in Chief Dr S.C. Joshi IFS (Retd) Chairman, Kerala State Biodiversity Board, Thiruvananthapuram Editorial team Dr. V. Balakrishnan Member Secretary, Kerala State Biodiversity Board Dr. Preetha N. Mrs. Mithrambika N. B. Dr. Baiju Lal B. Dr .Pradeep S. Dr . Suresh T. Mrs. Sunitha Menon Typography : Mrs. Ajmi U.R. Design: Shinelal Published by Kerala State Biodiversity Board, Thiruvananthapuram 2 FOREWORD Kerala is the only state in India where Biodiversity Management Committees (BMC) has been constituted in all Panchayats, Municipalities and Corporation way back in 2012. The BMCs of Kerala has also been declared as Environmental watch groups by the Government of Kerala vide GO No 04/13/Envt dated 13.05.2013. In Kerala after the devastating natural disasters of August 2018 Post Disaster Needs Assessment ( PDNA) has been conducted officially by international organizations. The present report of Rapid Impact Assessment of flood/ landslides on Biodiversity focus on community perspectives of the affect on Biodiversity and Ecosystems. It is for the first time in India that such an assessment of impact of natural disasters on Biodiversity was conducted at LSG level and it is a collaborative effort of BMC and Kerala State Biodiversity Board (KSBB). More importantly each of the 187 BMCs who were involved had also outlined the major causes for such an impact as perceived by them and suggested strategies for biodiversity conservation at local level. Being a study conducted by local community all efforts has been made to incorporate practical approaches for prioritizing areas for biodiversity conservation which can be implemented at local level. -

River Water Mercury Content Analysis at Palakkad District and the Design of Mercury Adsorbing Cfl Disposal System
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 02 Issue: 05 | Aug-2015 www.irjet.net p-ISSN: 2395-0072 RIVER WATER MERCURY CONTENT ANALYSIS AT PALAKKAD DISTRICT AND THE DESIGN OF MERCURY ADSORBING CFL DISPOSAL SYSTEM Sreelakshmi K S1, Dr. P N Ramachandran2 1 M.Tech (Energy Systems), Department of Electrical and Electronics Engineering, NCERC, Kerala, India 2 HOD, Department of Electrical and Electronics Engineering, NCERC, Kerala, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract -Analysis of mercury content has been 1. INTRODUCTION conducted by taking samples from tributaries and sub Mercury is a very toxic element which can be found both as an introduced contaminant and naturally in the tributaries of Bharathapuzha river at Palakkad district. environment. Its high potential for toxicity was well Subsurface and bottom sediment samples were taken. documented in the highly contaminated areas of Minamata Temperature and pH of the samples were also noted. Bay, Japan in the 1950’s and 1960’s. Mercury can be a The analysis was conducted at Sophisticated Test and menace to people's health and wildlife in many Instrumentation Centre lab at Ernakulam. The environments that are not discernibly polluted. The risk is instrument used for the analysis was Hydra C direct determined by the form of mercury present, the likelihood mercury analyzer which works on the principle of of exposure and the ecological and geochemical factors that influence how mercury moves and changes form in thermal decomposition with Atomic Absorption the environment. Mercury’s toxic effects depends on the Spectroscopy. The analysis results showed that there route of exposure and its chemical form. -

Freshwater Fishes of Ker A
BIONOMICS, RESOURCE CHARACTERISTICS AND DISTRIBUTION OF THE THREATENED FRESHWATER FISHES OF KER A THESIS SUBMITTED TO THE COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY BY EUPHRASIA C. ]. SCHOOL OF INDUSTRIAL FISHERIES COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY KOCHI — 682016 2004 DECLARATION I, Euphrasia C.J., do hereby declare that the thesis entitled “BlONOMICS, RESOURCE CHARACTERISTICS AND DISTRIBUTION OF THE THREATENED FRESHWATER FISHES OF KERALA” is a genuine record of research work carried out by me under the guidance of Dr. B. Madhusoodana Kurup, Professor, School of Industrial Fisheries, Cochin University of Science and Technology, Kochi-16 and no part of the work has previously formed the basis for the award of any Degree, Associateship and Fellowship or any other similar title or recognition of any University or institution. Kochi — 682018 'aw. (2/7n_aL 30"‘ July 2004 EUPHRASIA c. J. CERTIFICATE This is to certify that the thesis entitled “BIONOMICS, RESOURCE CHARACTERISTICS AND DISTRIBUTION OF THE THREATENED FRESHWATER FISHES OF KERALA” is an authentic record of research work carried out by Mrs. Euphrasia C. J. under my guidance and supervision in the School of Industrial Fisheries, Cochin University of Science and Technology in partial fulfilment of the requirements for the degree of Doctor of Philosophy and no part thereof has been submitted for any other degree. Kochi — 682016 Dr. B. Madhusoodana Kurup 30”‘ July, 2004 (Supervising Guide) Professor School of Industrial Fisheries Cochin University of Science and Technology, Kochi — 16 I wish to express my profound sense of gratitude and indebtedness to my Supervising Guide Dr. -

Natural Resources Sand Mafia in India
NATURAL RESOURCES SAND MAFIA IN INDIA Dr Susan Bliss Educational consultant Author, Macmillan Australia Sand mining mafia near the Kandluru Bridge located in the Kundapur taluk Photo source: http://data1.ibtimes.co.in/cache-img-0-450/en/full/566918/1491207555_sand-mafia.jpg Geography Syllabus Links such as sand, stone and clay, for infrastructure projects to build new towns, skyscrapers, flyovers, airports and • Landscapes and Landforms: Humans change – increase number of highway lanes. rivers, coasts and ocean beds India’s Prime Minister Narendra Modi, plans to develop • Environmental Change (Stage 4) and 100 smart cities under a ‘new Chicago every year’ slogan. Management (Stage 5) The speed of construction is concerning. Does India • Urbanisation (Stage 5 & 6) have sufficient sand for this development? What will be • Interconnections (Stage 4) the impacts on environments? • Natural Resources (Stage 6) Sand mafia: dark secrets of India’s • Cross- curriculum priorities: Asia, Sustainability booming construction industry Sand dubbed India’s ‘new gold’ Illegal sand mining is everywhere. Laws and inaction contribute to problem The construction industry is India’s largest economic sector accounting for 7.8% of the country’s GDP and the The world is running low on sand and pillaging sand is second largest employer. The high rate of urbanisation a growing global practice. ‘The construction-building and urban growth has accelerated the growth of industry is the largest consumer of this finite resource. the construction industry especially in cities such as The traditional average-sized house requires 200 tons Mumbai accommodating 12.5 million inhabitants, and of sand; a hospital requires 3,000 tons of sand; each Delhi 11 million.