Properties of Natural Waxes Used in Dentistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
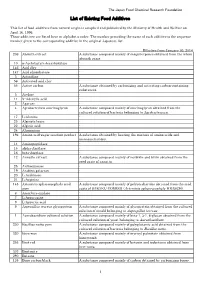
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Chemical and Technical Assessment 65Th JECFA
Chemical and Technical Assessment 65th JECFA BEESWAX Chemical and Technical Assessment (CTA) First draft prepared by Paul M. Kuznesof, Ph.D.∗ Reviewed by D. Brian Whitehouse, Ph.D. 1 Summary Beeswax (INS No. 901) consists primarily of a mixture of esters of fatty acids and fatty alcohols, paraffinic hydrocarbons, and free fatty acids; minor amounts of free fatty alcohols are also present. Two types of beeswax are marketed: yellow beeswax (C.A.S No. 8006-40-4) and white beeswax (C.A.S. No. 8012-89-3). Yellow beeswax is a yellow or light-brown solid that is somewhat brittle when cold and presents a characteristic odour of honey. White beeswax is a white or yellowish white solid (thin layers are translucent) having a characteristic, but faint, odour of honey. Beeswax is obtained from the honeycombs of bees (Apis mellifera L., Fam. Apidae) after removal of the honey. The combs are melted with hot water, steam, or solar heat. After removing the insoluble impurities, the liquid wax is cast into cakes for further purification to obtain food-grade yellow beeswax. Bleaching the latter with e.g. hydrogen peroxide, sulfuric acid or sunlight, yields white beeswax. Beeswax consists primarily of five main groups of components, namely: 1. Free fatty acids (typically 12-14%), most of which are saturated (ca. 85%) and have a chain length of C24-C32. 2. Free primary fatty alcohols (ca. 1%) with a chain length of C28-C35. 3. Linear wax monoesters and hydroxymonoesters (35-45%) with chain lengths generally of C40- C48. The esters are derived almost exclusively from palmitic acid, 15-hydroxypalmitic acid, and oleic acid. -

Sales Brochure Rev M 01A-8-15 FINAL BLU
Waxes Source Grades Colors Forms Uses & Special Features BEESWAX Honeycomb of the Crude, bleached, re- Cream-white to Cakes, slabs, pas- Color Cosmetics, Lip Care, Bee fined yellow, white, dark brown tilles, granular. Mascara, Eye Makeup, Hair cosmetic Wax, Creams, Lotions, Pharma- ceuticals, Ointments, Tablet Coatings, Confectionary Glazes, Candles. CANDELILLA Scales covering reed Crude, Refined, Light brown to Crude lumps, Color Cosmetics, Lip Care, like plants in Texas Bleached light yellow. refined lumps, Mascara, Eye Makeup, Hair and Mexico flakes, granular, & Wax, Pharmaceuticals, Tablet powder Coatings, Polishes, Precision Casting, Adhesives, Chewing Gum Base. CARNAUBA Exuded by the leaves No. 1 Yellow (Type 1) Yellow to Lumps, flakes, Color Cosmetics, Lip Care, of Brazilian "Tree of NC #3 Light (Type 3) brownish green. granular, & pow- Mascara, Eye Makeup, Pharma- Life" NC#3 Dark (Type 4- der ceuticals, Ointments, Tablet Filtered) Coatings, Confectionary Glazes, NC#3 Centrifuged Investment Casting, Polishes, , (Type 4) Inks and Thermal Transfer Rib- bon Coating, Fruit Wax Emul- sion. CERESINE & Formulated hydrocar- Specific to melting White Slabs, pastilles, & Color Cosmetics, Lip Care, OZOKERITE bons point, penetration, and granular. Mascara, Eye Makeup, Hair gel strength. Wax, Creams, Lotions, Pharma- ceuticals, Ointments, Coatings, Lubricants, Waterproofing, Adhesives. PARAFFIN & Petroleum Waxes Refined to Melt point White to Yellow Slabs, & pastilles. Cosmetics, Color Cosmetics, MICROCRYSTAL- & Color Lip Care, Mascara, Eye -

Celebrating the Rich History of Waxes Bladel, the Netherlands What’S Inside: Watertown, Connecticut, Usa
CELEBRATING THE RICH HISTORY OF WAXES BLADEL, THE NETHERLANDS WHAT’S INSIDE: WATERTOWN, CONNECTICUT, USA 2-3 – HERITAGE 4-5 – INNOVATION 6-7 – WORLD RESOURCES 8-9 – NATURAL/ORGANIC 10-11 – SILICONYL WAXES 12-13 – CUSTOM BLENDS 14-15 – EMULSIFYING WAXES 16-17 – KESTER WAXES 18-19 – MILKS 20-41 – WAX SPECIFICATIONS 42 – WAX PROPERTIES KOSTER WAX FACT: Koster Keunen was founded in the Netherlands and is world renowned for supplying quality waxes. 1852 OUR HISTORY OF TRADITION AND INNOVATION Founded in 1852 as a family business, Koster Keunen has evolved into the world’s leading processor, refiner and marketer of natural waxes. From the early days of sun bleaching beeswax for the candle industry, we now specialize in processing and formulating quality waxes for cosmetics, pharmaceutical, food, coatings, and various other technical industries worldwide. For over 150 years we have sought perfection, constantly introducing new and innovative processes and waxes, while investing in experienced, knowledgeable people and the best equipment to help meet this goal. As a family business we believe very strongly in the need for developing 3 superior quality products, and supporting our customers with excellent service, throughout the formulation and marketing processes. From our two facilities, in the USA and Holland, we offer a huge range of natural waxes, synthetic waxes and wax derivatives, enabling our customers to produce thousands of products that look, feel and work superbly KOSTERKEUNEN.COM / 1 860.945.3333 KOSTER WAX FACT: Koster Keunen was the first natural wax company to manufacture waxes using a Sandvik Pastillator, starting in 1988. 1852 UNIQUELY KOSTER KEUNEN Our greatest strength is the experience and scientific expertise we have fostered for the development of new and innovative products. -
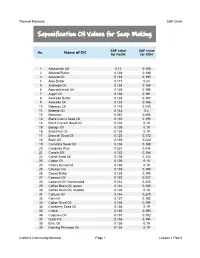
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

Some Flow Characteristics at 37 Degrees C of Ternary Wax Mixtures
NATIONAL BUREAU OF STANDARDS REPORT 9060 Progress Report on Some Flow Characteristics at 37°C of Ternary Wax Mixtures That May Have Possible Dental Uses <&> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and maintenance of the national stand- ards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particu- larly for government agencies; cooperation in the establishment of standard practices for incorpora- tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, tech- nical, and engineering information. The scope of the Bureau’s activities is suggested in the following listing of its four Institutes and their organizational units. Institute for Basic Standards. Applied Mathematics. Electricity. Metrology. Mechanics. Heat. Atomic Physics. Physical Chemistry. Laboratory Astrophysics.* Radiation Physics. Radio Standards Laboratory:* Radio Standards Physics; Radio Standards Engineering. -

Synthesis and Properties of a Lacquer Wax-Based Quarternary Ammonium Gemini Surfactant
Molecules 2014, 19, 3596-3606; doi:10.3390/molecules19033596 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Synthesis and Properties of a Lacquer Wax-Based Quarternary Ammonium Gemini Surfactant Hongxia Chen 1, Chengzhang Wang 1,2,*, Jianzhong Ye 1, Hao Zhou 1,2, Li Lu 3 and Zhibing Yang 3 1 Institute of Chemical Industry of Forest Products, CAF, National Engineering Lab for Biomass Chemical Utilization; Key and Open Laboratory of Forest Chemical Engineering, SFA, Jiangsu Province, Nanjing 210042, China 2 Institute of New Technology of Forestry, CAF, Beijing 100091, China 3 Hubei Academy of Forestry, Wuhan, 430075, China * Author to whom correspondence should be addressed; E-Mails: [email protected] or [email protected]; Tel./Fax: +86-025-8548-2471. Received: 4 November 2013; in revised form: 11 March 2014 / Accepted: 14 March 2014 / Published: 24 March 2014 Abstract: Lacquer wax is an important fatty resource obtained from the mesocarp of the berries of Toxicodendron vernicifluum. In order to expand the applications of lacquer wax, we hydrolyzed it after establishing the best conditions for the acid-catalyzed hydrolysis using a Box-Behnken design. Then we synthesized a quarternary ammonium gemini surfactant by a three-step reaction. The surface properties of an aqueous solution of the final product were investigated. The optimum conditions were 9% catalyst, 100 °C of reaction temperature and 14 h of reaction time, while the maximum free fatty acids (FFA)% was 99.67%. From the gas chromatography, the main fatty acids of the lacquer wax were palmitic, oleic and octadecanoic acid. The lacquer wax gemini surfactant was synthesized, and its structure was confirmed by IR and NMR. -

Drug Adulteration: a Threat to Efficacy of NAAS Rating 2017: 3.53 JMPS 2017; 5(4): 01-06 Ayurveda Medicine © 2017 JMPS Received: 01-05-2017 Accepted: 02-06-2017 Dr
Journal of Medicinal Plants Studies 2017; 5(4): 01-06 ISSN (E): 2320-3862 ISSN (P): 2394-0530 Drug adulteration: A threat to efficacy of NAAS Rating 2017: 3.53 JMPS 2017; 5(4): 01-06 ayurveda medicine © 2017 JMPS Received: 01-05-2017 Accepted: 02-06-2017 Dr. Sreelekshmi M, Dr. Vimala KS, Dr. Raiby P Paul, Dr. Nidhin Dr. Sreelekshmi M Chandran, Dr. Priyanka A Shine and Dr. Nazneen A Salam PG Scholar, Department of Dravyaguna, School of Ayurveda, Amrita University, Abstract Kollam, Kerala, India Even though Ayurveda has gained popularity among the medicinal systems there is a hindrance to its further development. One of the burning problems is the practice of adulteration which creates doubts Dr. Vimala KS and disbelief in the curative capability of traditional systems.In the present work methods & reasons for Professor, Department of adulteration are described. The efficacies of Ayurvedic Products are critically dependent on an Dravyaguna, School of uninterrupted availability of herbs. The unethical practice of adulteration by the drug manufacturers Ayurveda, Amrita University, would not only reduce the efficacy of the drugs but also affect the trust of the people in the traditional Kollam, Kerala, India healthcare systems. Adulteration may be evaluated by different methods like morphological or Dr. Raiby P Paul organoleptic tests, microscopic evaluation, chemical evaluation, physical evaluation, chromatography, Assistant Professor, Department spectrophotometry etc. The solution lies in ensuring the availability of crude drugs. Studies on of Dravyaguna, School of adulteration practices will have to be taken up along with identification of the scares drugs. Conservation Ayurveda, Amrita University, measures of their natural habitat & ex-situ medicinal plant cultivation may have to be taken up in large Kollam, Kerala, India scale. -

The Use of Particular Natural Oils in Formulations
Tony O’Lenick – Siltech LLC, US WAXES AND BUTTERS The use of particular natural oils in formulations ABSTRACT CH2-OH O CH2-OC(O)-R Natural oils have become a common additive to personal care products. They CH-OH +3 RC-OH CH-OC(O)-R + 3 H2O fit the definition of natural, sustainable and can be organic and even organic. CH OH CH -O-C(O)-R While these classifications are 2 2 sometimes unclear in meaning, the reason we formulate with a particular oil Glycerin Fatty acid Triglyceride Water is often just as obscure. Is it marketing, or carbon distribution or aesthetics or Figure 1: The reaction. perhaps a particular benefit rendered to the oil by a particular native material The selection of a particular natural oil in triglycerides. Fats have a titer point of (antioxidants)? This article will look at a personal care application depends upon over 40.5˚C, oils have a titer point of some of these properties. what the formulator wants to achieve with below 40.5˚C. Butters have a titer below the particular formulation. The simplest 40.5˚C but above 20˚C. Oils are liquid choice is when the choice is simply a at room temperature and we now use results in a very wide melting ‘range’ for marketing choice. The product profile this word to describe any compound these compounds. For this reason, titer may say that the product should contain that is a liquid and is insoluble in water. point is generally determined on fats, oils, a particular oil like olive oil or argan oil. -

Food and Drug Administration, HHS § 186.1756
Food and Drug Administration, HHS Pt. 186 It is produced commercially by extrac- tion on the availability of this mate- tion from corn gluten with alkaline rial at NARA, call 202–741–6030, or go aqueous isopropyl alcohol containing to: http://www.archives.gov/ sodium hydroxide. The extract is then federallregister/ cooled, which causes the zein to pre- codeloflfederallregulations/ cipitate. ibrllocations.html. (b) The ingredient must be of a pu- (c) In accordance with § 184.1(b)(1), rity suitable for its intended use. the ingredient is used in food with no (c) In accordance with § 184.1(b)(1), limitations other than current good the ingredient is used in food with no manufacturing practice. The affirma- limitation other than current good tion of this ingredient as generally rec- manufacturing practice. The affirma- ognized as safe as a direct human food tion of this ingredient as generally rec- ingredient is based upon the following ognized as safe (GRAS) as a direct current good manufacturing practice human food ingredient is based upon conditions of use: the following current good manufac- (1) The ingredient is used as an en- turing practice conditions of use: zyme, as defined in § 170.3(o)(9) of this (1) The ingredient is used as a sur- chapter, as an optional ingredient for face-finishing agent as defined in flavor development in the manufacture § 170.3(o)(30) of this chapter. of cheddar cheese, in accordance with (2) The ingredient is used in food at § 133.113 of this chapter, and in the levels not to exceed current good man- preparation of protein hydrolysates. -

300 Carnauba Wax Wood Finish
N a t u r a l C h e m i s t r y P r o d u c t s EE C COO -- SSEE s # 300 UU roduct OO istry P HH l Chem Natura Carnauba Wax Wood Finish For Hardwood, Softwood, Cork & Stone Surfaces Technical Satin ‘ Wear & Tear ’ finish coat for oiled and untreated surfaces Data Sheet clear transparent , water-resistent Application Range : indoors only : Use on most types of fine wood surfaces, which are previously oiled or untreated. Enhances the wood grain and increases the resistance of wood surfaces against abrasion, water and dirt penetration. Also suitable for natural stone and unglazed tiles. Easy to clean, just use a mild soap and water. Worn patches can be restored with additional coats. Do not use in frequently wet areas. Technical Properties : Ideal for furniture, architectural woodwork and floors with smooth surfaces. Creates an elastic, breathable, anti-static protective coating. Water-resistent. Mode of Application : Surfaces must be smooth, free of dust and clean. Apply thin coat by cotton cloth or wax brush, ideally parallel with the wood grain. Avoid build-ups in cavities or cracks. In such cases remove excess material, if already hardened with # 115 Xtra Mild Citrus Thinner. Let dry for 48 hours or longer ( ideally 4 - 7 days ), then buff to a sheen with a wax buffer or a buffing machine applying strong pressure. We recommend to buff again on the following day. Do not use floor between waxing and buffing. Use moderately and do not clean wet during the first 3 weeks. -

Tall Oil. Bon Chain Length Are 15 to 30 Percent (A) Tall Oil (CAS Reg
§ 186.1555 21 CFR Ch. I (4–1–13 Edition) percentages of total fatty acids by car- § 186.1557 Tall oil. bon chain length are 15 to 30 percent (a) Tall oil (CAS Reg. No. 8002–26–4) is each of C , C , C , C , less than 10 16 18 20 22 essentially the sap of the pine tree. It percent C or lower carbon chain 14 is obtained commercially from the length, and less than 1 percent C or 24 waste liquors of pinewood pulp mills higher carbon chain length fatty acids. and consists mainly of tall oil resin (c) The ingredient is used as a con- acids and tall oil fatty acids. stituent of cotton and cotton fabrics used for dry food packaging. (b) In accordance with § 186.1(b)(1), the ingredient is used as an indirect (d) The ingredient is used at levels human food ingredient with no limita- not to exceed good manufacturing tion other than current good manufac- practice in accordance with § 186.1(b)(1). turing practice. The affirmation of this (e) Prior sanctions for this ingredient ingredient as generally recognized as different from the use established in safe (GRAS) as an indirect human food this section do not exist or have been ingredient is based on the following waived. current good manufacturing practice [44 FR 28323, May 15, 1979, as amended at 49 conditions of use: FR 5614, Feb. 14, 1984; 58 FR 17099, Apr. 1, (1) The ingredient is used as a con- 1993] stituent of cotton and cotton fabrics used for dry food packaging.