Carbon Dioxide Mixtures As Working Fluid for High-Temperature Heat Recovery: a Thermodynamic Comparison with Transcritical Organic Rankine Cycles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Performance Analysis of Two-Stage Transcritical Refrigeration Cycle Operating with R744
Proceedings of COBEM 2011 21 st Brazilian Congress of Mechanical Engineering Copyright © 2011 by ABCM October 24-28, 2011, Natal, RN, Brazil PERFORMANCE ANALYSIS OF TWO-STAGE TRANSCRITICAL REFRIGERATION CYCLE OPERATING WITH R744 Igor Marcel Gomes Almeida, [email protected] Studies Group in Refrigeration and Air-Conditioning. Federal Institute of Education, Science and Technology of Rio Grande do Norte. Santa Cruz-RN. Cleiton Rubens Formiga Barbosa, [email protected] Francisco de Assis Oliveira Fontes, [email protected] Federal University of Rio Grande do Norte. Department of Mechanical Engineering. Abstract. The present study is a theoretical analysis of a two-stage transcritical cooling cycle using R744 (carbon dioxide) as a refrigerant. The effect of the intercooling process on performance in the two-stage transcritical system is subjected to varying pressures in a gas cooler. Performance comparison between the single stage and two-stage cycle is also subject to the same operating conditions. We assessed the effects on system performance of pressure in the gas cooler, isentropic compressor efficiency, amount of intercooling between the two compression stages and discharge temperature of the refrigerant from the gas cooler. Thermodynamic analysis was carried out with CoolPack software, considering an evaporation temperature of -10 oC and refrigeration capacity of 5 kW. Results show the coefficient of performance (COP) for the two-stage cycle is superior to the single stage cycle. Two-stage compression was found to reduce energy consumption. On the whole, two-stage compression and the intercooling process are significant in increasing COP for these systems. Keywords : Natural refrigerants, Carbon dioxide, Performance, Two-stage compression, Transcritical cycle. -

Performance Comparison of Different Rankine Cycle Technologies Applied to Low and Medium Temperature Industrial Surplus Heat Scenarios
Paper ID: 175 Page 1 Performance Comparison of Different Rankine Cycle Technologies Applied to Low and Medium Temperature Industrial Surplus Heat Scenarios Goran Durakovic1*, Monika Nikolaisen2 SINTEF Energy Research, Gas technology, Trondheim, Norway [email protected] [email protected] * Corresponding Author ABSTRACT Energy intensive industries generate vast amounts of low-grade surplus heat, and potential for direct re- use can be limited due to insufficient local demand. Conversion of heat into electric power thus becomes an attractive option. However, heat-to-power conversion is limited by poor efficiencies for heat sources at low and medium temperatures. To optimally utilize the available energy, the choice of technology could be important. In this work, single-stage subcritical, dual-stage subcritical and single-stage transcritical Rankine cycle technologies were compared. The investigated heat sources are an air equivalent off-gas at 150°C and 200°C and a liquid heat source at 150°C. These were all evaluated with the same available duty. The Rankine cycles were compared with equal total heat exchanger areas to enable fair comparisons of different cycles, instead of comparing cycles based on equal pinch point temperature differences as is frequently reported in literature. Six working fluids were investigated for both heat source temperatures, and cycle optimization was performed to determine the best Rankine cycle for each working fluid. This means that the optimizer could choose between single-stage and dual-stage cycle during one optimization, and determine which gave the highest net power output. The results were benchmarked against single-stage subcritical Rankine cycles. The results showed that the cycle yielding the highest net power varied depending on the allowable total heat exchanger area. -

Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho a Dissertation
Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems By Tony Ho A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering – Mechanical Engineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair Professor Van P. Carey Professor Per F. Peterson Spring 2012 Abstract Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho Doctor of Philosophy in Mechanical Engineering University of California, Berkeley Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially increase power generation and improve the utilization efficiency of renewable energy and waste heat recovery systems. A brief review of current advanced vapor power cycles including the Organic Rankine Cycle (ORC), the zeotropic Rankine cycle, the Kalina cycle, the transcritical cycle, and the trilateral flash cycle is presented. The premise and motivation for the OFC concept is that essentially by improving temperature matching to the energy reservoir stream during heat addition to the power cycle, less irreversibilities are generated and more power can be produced from a given finite thermal energy reservoir. In this study, modern equations of state explicit in Helmholtz energy such as the BACKONE equations, multi-parameter Span- Wagner equations, and the equations compiled in NIST REFPROP 8.0 were used to accurately determine thermodynamic property data for the working fluids considered. Though these equations of state tend to be significantly more complex than cubic equations both in form and computational schemes, modern Helmholtz equations provide much higher accuracy in the high pressure regions, liquid regions, and two-phase regions and also can be extended to accurately describe complex polar fluids. -

Analysis of a CO2 Transcritical Refrigeration Cycle with a Vortex Tube Expansion
sustainability Article Analysis of a CO2 Transcritical Refrigeration Cycle with a Vortex Tube Expansion Yefeng Liu *, Ying Sun and Danping Tang University of Shanghai for Science and Technology, Shanghai 200093, China; [email protected] (Y.S.); [email protected] (D.T.) * Correspondence: [email protected]; Tel.: +86-21-55272320; Fax: +86-21-55272376 Received: 25 January 2019; Accepted: 19 March 2019; Published: 4 April 2019 Abstract: A carbon dioxide (CO2) refrigeration system in a transcritical cycle requires modifications to improve the coefficient of performance (COP) for energy saving. This modification has become more important with the system’s more and more widely used applications in heat pump water heaters, automotive air conditioning, and space heating. In this paper, a single vortex tube is proposed to replace the expansion valve of a traditional CO2 transcritical refrigeration system to reduce irreversible loss and improve the COP. The principle of the proposed system is introduced and analyzed: Its mathematical model was developed to simulate and compare the system performance to the traditional system. The results showed that the proposed system could save energy, and the vortex tube inlet temperature and discharge pressure had significant impacts on COP improvement. When the vortex tube inlet temperature was 45 ◦C, and the discharge pressure was 9 MPa, the COP increased 33.7%. When the isentropic efficiency or cold mass fraction of the vortex tube increased, the COP increased about 10%. When the evaporation temperature or the cooling water inlet temperature of the desuperheater decreased, the COP also could increase about 10%. The optimal discharge pressure correlation of the proposed system was established, and its influences on COP improvement are discussed. -

Energy Efficiency State of the Art of Available Low-Gwprefrigerants And
ENERGY EFFICIENCY STATE OF THE ART OF AVAILABLE LOW-GWP REFRIGERANTS AND SYSTEMS Final Report September 2018 Study commissioned by the AFCE and carried out by EReIE, the Cemafroid, and the CITEPA Authors : Stéphanie Barrault (CITEPA) Olivier Calmels (Cemafroid) Denis Clodic (EReIE) Thomas Michineau (Cemafroid) ACKNOWLEDGEMENTS We sincerely thank the members of the Steering Committee for their contributions: Bénédicte BALLOT-MIGUET, EDF Emmanuelle BRIERE, UNICLIMA Tristan HUBE, Service Entreprises et Dynamiques Industrielles, ADEME Laurent GUEGAN, François HEYNDRICKX, Régis LEPORTIER, François PIGNARD, AFCE. We would also like to thank all the professionals contacted as part of the study Copyright 2018 Any representation or reproduction in whole or in part without the consent of the authors or their heirs or successors is illegal according to the Code of Intellectual Property (Art. L 122-4) and constitutes an infringement punishable under the Criminal Code. Only are allowed (art. 122-5) copies or reproductions strictly reserved for private use and not intended for collective use, as well as analyzes and short quotations justified by the critical pedagogical character, or information the work to which they are incorporated, subject, however, to compliance with the provisions of Articles L122-10 to L122-12 of the Code, relating to reprographic reproduction. 2 / 131 Content CONTENT Content 3 List of Figures _________________________________________________________________ 6 List of Tables _________________________________________________________________ -

Hermetic Gas Fired Residential Heat Pump
HERMETIC GAS FIRED RESIDENTIAL HEAT PUMP David M. Berchowitz, Director and Yong-Rak Kwon, Senior Engineer Global Cooling BV, Ijsselburcht 3, 6825 BS Arnhem, The Netherlands Tel: 31-(0)26-3653431, Fax: 31-(0)26-3653549, E-mail: [email protected] ABSTRACT The free-piston Stirling engine driven heat pump (FPSHP) is presented as an alternative residential heat pump technology. In this type of heat pump system the mechanical output of an externally heated free-piston Stirling engine (FPSE) is directly connected to a Rankine or transcritical cycle heat pump by way of a common piston assembly. The attractiveness of this system is the economics of operation when compared to an electrically driven conventional heat pump as well as the low environmental impact of the system. It is expected that the primary energy ratio for the ground water source FPSHP will be close to 2.15 for heating mode and 3.34 for cooling mode with the inclusion of domestic hot water generation. The working fluids are dominantly helium (He) gas for the engine and carbon dioxide (CO2) for the heat pump. Technical concerns for this system include the effects of working medium mixing and the load stability under various operating conditions. The direct connection of the Stirling engine to the compressor of the heat pump allows for the working fluids to mix with each other. He separation for the heat pump is discussed and the effect of the mixing of working fluids on both the heat pump and Stirling engine is investigated through a demonstrative experiment and simulation. Experimental verification of the performance due to the mixed working fluid is presented for the heat pump cycle, while simulation techniques with proper gas mixture properties are used to determine the effect on the Stirling cycle. -

Thermodynamic Comparison of Subcritical and Transcritical Heat Pump Water Heater (HPWH) Efficiency
CO2 vs. Fluorocarbons: Thermodynamic Comparison of Subcritical and Transcritical Heat Pump Water Heater (HPWH) Efficiency Kyle R. Gluesenkamp Kashif Nawaz ACEEE Hot Water Forum Nashville, March 13, 2019 Session 4A ORNL is managed by UT-Battelle, LLC for the US Department of Energy Acknowledgments • Department of Energy (Contract DE-EE0006718.00) • Antonio Bouza, DOE Building Technology Office DISCLAIMER This material has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan). 2 Classification of HPWHs Pumped (or external): Wrap-around: Immersed coil: HX Evaporator Evaporator Evaporator 3 Classification of Stratification Types (Stratification During Heating) Table 1. Tank Stratification Categories Heat exchanger (HX) Locations of tank High pressure used to transfer water taps, OR Stratifying Category refrigerant Water or refrigerant flow type refrigerant heat to coil vertical span potential glide water 1 Water pumped to HX Bottom and top Any Single pass (low -

Natural Refrigerant-Based Subcritical and Transcritical Cycles for High Temperature Heating Cycles Souscritiques Et Transcritiqu
International Journal of Refrigeration 30 (2007) 3e10 www.elsevier.com/locate/ijrefrig Natural refrigerant-based subcritical and transcritical cycles for high temperature heating J. Sarkar, Souvik Bhattacharyya*, M. Ram Gopal Department of Mechanical Engineering, Indian Institute of Technology, Kharagpur 721302, India Received 11 April 2005; received in revised form 31 January 2006; accepted 13 March 2006 Available online 5 June 2006 Abstract Theoretical analyses of subcritical/transcritical heat pumps using four natural refrigerants, carbon dioxide, ammonia, pro- pane and isobutane have been carried out for high temperature heating applications at different heating outlet temperatures and heat sources using computer models. The compressor discharge pressures have been optimized for transcritical and subcrit- ical (with near critical operation of condenser) cycles. Results show that for subcritical heat pumps, use of subcooling is efficient for heating applications with a gliding temperature. Results also show that although propane yields better coefficient of perfor- mance (COP) in low temperature heating applications, ammonia performs the best in high temperature heating applications. Finally, design aspects of major components of all the four heat pumps for high temperature heating have been discussed, par- ticularly with reference to suitability of available lubricants to the newly evolved operating conditions. Ó 2006 Elsevier Ltd and IIR. All rights reserved. Keywords: Heat pump; Carbon dioxide; Ammonia; Propane; Isobutane; Research; Thermodynamic cycle; Transcritical cycle; Performance; COP Cycles souscritiques et transcritiques utilisant des frigorige`nes naturels dans les applications a` tempe´rature e´leve´e Mots cle´s : Pompe a` chaleur ; Dioxyde de carbone ; Ammoniac ; Propane ; Isobutane ; Recherche ; Cycle thermodynamique ; Cycle transcritique ; Performance ; COP 1. -

Comparison of the Organic Flash Cycle (OFC) to Other Advanced Vapor Cycles for Intermediate and High Temperature Waste Heat Reclamation and Solar Thermal Energy
Energy 42 (2012) 213e223 Contents lists available at SciVerse ScienceDirect Energy journal homepage: www.elsevier.com/locate/energy Comparison of the Organic Flash Cycle (OFC) to other advanced vapor cycles for intermediate and high temperature waste heat reclamation and solar thermal energy Tony Ho*, Samuel S. Mao, Ralph Greif Department of Mechanical Engineering, University of California-Berkeley, Etcheverry Hall, Berkeley, CA 94720, USA article info abstract Article history: The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially improve the Received 27 August 2011 efficiency with which high and intermediate temperature finite thermal sources are utilized. The OFC’s Received in revised form aim is to improve temperature matching and reduce exergy losses during heat addition. A theoretical 22 February 2012 investigation is conducted using high accuracy equations of state such as BACKONE, SpaneWagner, and Accepted 24 March 2012 REFPROP in a detailed thermodynamic and exergetic analysis. The study examines 10 different aromatic Available online 22 April 2012 hydrocarbons and siloxanes as potential working fluids. Comparisons are drawn between the OFC and an optimized basic Organic Rankine Cycle (ORC), a zeotropic Rankine cycle using a binary ammonia-water Keywords: Organic Rankine Cycle (ORC) mixture, and a transcritical CO2 cycle. Results showed aromatic hydrocarbons to be the better suited fl Solar thermal working uid for the ORC and OFC due to higher power output and less complex turbine designs. Results Waste heat also showed that the single flash OFC achieves comparable utilization efficiencies to the optimized basic Vapor cycle ORC. Although the OFC improved heat addition exergetic efficiency, this advantage was negated by Exergy analysis irreversibilities introduced during flash evaporation. -
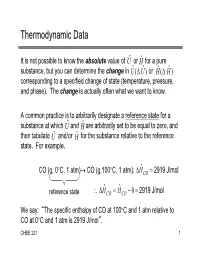
Thermodynamic Data
Thermodynamic Data It is not possible to know the absolute value of U ˆ or H ˆ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or Hˆ ( Hˆ ) corresponding to a specified change of state (temperature, pressure, and phase). The change is actually often what we want to know. A common practice is to arbitrarily designate a reference state for a substance at which U ˆ and H ˆ are arbitrarily set to be equal to zero, and then tabulate U ˆ and/or H ˆ for the substance relative to the reference state. For example, ˆ CO (g, 0C, 1 atm) CO (g,100C, 1 atm): HCO 2919 J/mol ˆ ˆ reference state HCO HCO 0 2919 J/mol We say: “The specific enthalpy of CO at 100C and 1 atm relative to CO at 0C and 1 atm is 2919 J/mol”. CHEE 221 1 Reference States and State Properties Most (all?) enthalpy tables report the reference states (T, P and State) on which the values of H ˆ are based; however, it is not necessary to know the reference state to calculate H (change in enthalpy) for the transition from one state to another. ˆ ˆ –H from state 1 to state 2 equals H 2 H 1 regardless of the reference state upon which ˆ and ˆ were based H1 H 2 – Caution: If you use different tables, you must make sure they have the same reference state This result is a consequence of the fact that H ˆ (and U ˆ ) are state properties, that is, their values depend only on the state of the species (temperature, pressure, state) and not on how the species reached its state. -

Non-Dimensional Parameter Development for Transcritical Cycles H
Purdue University Purdue e-Pubs International Refrigeration and Air Conditioning School of Mechanical Engineering Conference 2002 Non-Dimensional Parameter Development For Transcritical Cycles H. E. Howard Praxair Follow this and additional works at: http://docs.lib.purdue.edu/iracc Howard, H. E., "Non-Dimensional Parameter Development For Transcritical Cycles" (2002). International Refrigeration and Air Conditioning Conference. Paper 579. http://docs.lib.purdue.edu/iracc/579 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Complete proceedings may be acquired in print and on CD-ROM directly from the Ray W. Herrick Laboratories at https://engineering.purdue.edu/ Herrick/Events/orderlit.html R11-3 Non-Dimensional Parameter Development for Transcritical Cycles Henry Howard, Sr. Development Associate, Praxair, Inc. 175 East Park Drive, P.O. Box 44 Tonawanda, NY 14150-7891, USA; Tel.: 716/879-2764; Fax: 716/879-7567 E-Mail: [email protected] ABSTRACT Increased commercial interest with regard to carbon dioxide’s use in transcritical cycles has led to numerous modifications to the basic vapor compression cycle. The transcritical refrigeration cycle is characterized by the fact that supercritical high-side pressure is an optimization variable. Most cycle adaptations have been conceived with the intent of facilitating optimal high side pressure control. Depending upon operating range, improper high- side pressure definition may lead to power penalties in excess of 10%. A critical element in system design and optimization involves the mechanism for dynamic computation of the optimal high side pressure. Common approaches to this problem involve heuristics and empirical correlation. -

Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations
Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations A 1st Law, 2nd Law and Economic Analysis of the Practical Steam Engine in a District Energy/CHP Application Joshua A. Tolbert, Ph.D. Practical Steam Energy Efficiency Engineering – How is efficiency calculated? • What is the Status Quo? – Engineers currently focus on limiting energy losses as the primary point of focus for district energy and CHP systems – Only local energy losses are typically considered causing energy efficiency opportunities to be wasted • Why? – Conventional wisdom tells us that minimizing local energy losses is ultimate goal • How should we change? – Could a different approach improve global efficiency? Motivation Common Questions: 1. How can installing an imperfect device in parallel to an isenthalpic pressure reducing valve (PRV) improve efficiency? 2. My analysis shows an incremental fuel cost with PRV parallel, how can this be more efficient? Analysis Overview • Consider a Practical Steam Engine (PSE) in a Pressure Reducing Valve (PRV) parallel district energy application • PSE operation is consistent with CHP application • Consider 1st Law, 2nd Law and economic analysis for energy efficiency • Draw conclusions Backpressure Application – Case Study Enwave Seattle - District Energy / CHP Application Assumptions Equipment • 1.5 MW (5.118 MMBtu/hr) • Practical Steam Engine (PSE) heating load is considered o Isentropic efficiency of 80% • Condensate exiting load: o Mechanical efficiency of 80% Saturate liquid at 20 psig o Generator efficiency of 95% • No heat losses in piping or • Isenthalpic PRV equipment* • 150 psig saturated steam boiler • 80% boiler and feedwater pump efficiency *Incorporating actual heat losses does not significantly affect results of analysis.