Corticosteroids ELISA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmaceutical and Veterinary Compounds and Metabolites
PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES High quality reference materials for analytical testing of pharmaceutical and veterinary compounds and metabolites. lgcstandards.com/drehrenstorfer [email protected] LGC Quality | ISO 17034 | ISO/IEC 17025 | ISO 9001 PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES What you need to know Pharmaceutical and veterinary medicines are essential for To facilitate the fair trade of food, and to ensure a consistent human and animal welfare, but their use can leave residues and evidence-based approach to consumer protection across in both the food chain and the environment. In a 2019 survey the globe, the Codex Alimentarius Commission (“Codex”) was of EU member states, the European Food Safety Authority established in 1963. Codex is a joint agency of the FAO (Food (EFSA) found that the number one food safety concern was and Agriculture Office of the United Nations) and the WHO the misuse of antibiotics, hormones and steroids in farm (World Health Organisation). It is responsible for producing animals. This is, in part, related to the issue of growing antibiotic and maintaining the Codex Alimentarius: a compendium of resistance in humans as a result of their potential overuse in standards, guidelines and codes of practice relating to food animals. This level of concern and increasing awareness of safety. The legal framework for the authorisation, distribution the risks associated with veterinary residues entering the food and control of Veterinary Medicinal Products (VMPs) varies chain has led to many regulatory bodies increasing surveillance from country to country, but certain common principles activities for pharmaceutical and veterinary residues in food and apply which are described in the Codex guidelines. -

Treatment Processes for Microbial Resistance Mitigation: the Technological Contribution to Tackle the Problem of Antibiotic Resistance
International Journal of Environmental Research and Public Health Review Treatment Processes for Microbial Resistance Mitigation: The Technological Contribution to Tackle the Problem of Antibiotic Resistance Gabriela Bairán 1, Georgette Rebollar-Pérez 2, Edith Chávez-Bravo 1,* and Eduardo Torres 1,* 1 Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla, Puebla 72570, Mexico; [email protected] 2 Facultad de Ingeniería Química, Benemérita Universidad Autónoma de Puebla, Puebla 72570, Mexico; [email protected] * Correspondence: [email protected] (E.C.-B.); [email protected] (E.T.) Received: 24 September 2020; Accepted: 24 November 2020; Published: 28 November 2020 Abstract: Advances generated in medicine, science, and technology have contributed to a better quality of life in recent years; however, antimicrobial resistance has also benefited from these advances, creating various environmental and health problems. Several determinants may explain the problem of antimicrobial resistance, such as wastewater treatment plants that represent a powerful agent for the promotion of antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARG), and are an important factor in mitigating the problem. This article focuses on reviewing current technologies for ARB and ARG removal treatments, which include disinfection, constructed wetlands, advanced oxidation processes (AOP), anaerobic, aerobic, or combined treatments, and nanomaterial-based treatments. Some of these technologies are highly intensive, such as AOP; however, other technologies require long treatment times or high doses of oxidizing agents. From this review, it can be concluded that treatment technologies must be significantly enhanced before the environmental and heath problems associated with antimicrobial resistance can be effectively solved. -

( 12 ) United States Patent ( 10 ) Patent No .: US 10,835,538 B2 Averback ( 45 ) Date of Patent : Nov
US010835538B2 ( 12 ) United States Patent ( 10 ) Patent No .: US 10,835,538 B2 Averback ( 45 ) Date of Patent : Nov. 17 , 2020 ( 54 ) METHOD OF TREATING BENIGN 7,241,738 B2 7/2007 Averback et al . 7,317,077 B2 1/2008 Averback et al . PROSTATIC HYPERLASIA WITH 7,408,021 B2 8/2008 Averback et al . ANTIBIOTICS 7,745,572 B2 6/2010 Averback et al . 8,067,378 B2 11/2011 Averback et al . ( 71 ) Applicant: Nymox Corporation , Nassau ( BS ) 8,293,703 B2 10/2012 Averback et al . 8,569,446 B2 10/2013 Averback et al . 8,716,247 B2 5/2014 Averback et al . ( 72 ) Inventor : Paul Averback , Nassau ( BS ) 2016/0215031 Al 7/2016 Averback 2016/0361380 A1 12/2016 Averback ( 73 ) Assignee : NYMOX CORPORATION , Nassau 2017/0020957 Al 1/2017 Averback ( BS ) 2017/0360885 Al 12/2017 Averback 2018/0064785 Al 3/2018 Averback ( * ) Notice : Subject to any disclaimer , the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154 ( b ) by 21 days. WO 90/08555 A1 8/1990 WO WO - 2007149312 A2 * 12/2007 A61K 8/606 ( 21 ) Appl. No .: 15 /938,920 ( 22 ) Filed : Mar. 28 , 2018 OTHER PUBLICATIONS McClellan et al. , Topical Metronidazole A Review of its Use in ( 65 ) Prior Publication Data Rosacea, 2000 , Am J Clin Dermatol, 1 ( 3 ) , pp . 191-199 ( Year: 2000 ) . * US 2019/0298731 A1 Oct. 3 , 2019 Ricciotti et al . , Prostaglandins and Inflammation, 2011 , Arterioscler Thromb Vasc Biol . , 31 ( 5 ) , pp . 986-1000 ( Year: 2011 ) . * ( 51 ) Int . -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2019 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -
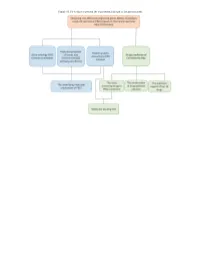
Figure S1. Flow Chart to Present the Experimental Design of the Present Study
Figure S1. Flow chart to present the experimental design of the present study. Figure S2. Volcano plot showing the differentially expressed genes between papillary renal cell carcinoma and non-cancer adjacent samples. Figure S3. The 2D molecular structures of the top 10 drugs. Table SI. The 60 potential drugs for papillary renal cell carcinoma treatment based on differently expressed genes. Drug name Dose Cell line Score Instance ID Pinacidil (C13H19N5) 16 µM HL60 -1 2406 Ciclosporin (C62H111N11O12) 3 µM HL60 -0.994 1331 Naftifine (C21H21N) 12 µM HL60 -0.966 2974 Vorinostat (C14H20N2O3) 10 µM HL60 -0.961 1161 Metacycline (C22H22N2O8) 8 µM HL60 -0.943 2901 Sulfacetamide (C8H10N2O3S) 16 µM HL60 -0.939 1859 Chlorprothixene (C18H18ClNS) 11 µM HL60 -0.938 1272 Amiodarone (C25H29I2NO3) 6 µM HL60 -0.933 2434 Noretynodrel (C20H26O2) 13 µM HL60 -0.914 1860 Valproic acid (C8H16O2) 200 µM HL60 -0.91 1155 Oxolinic acid (C13H11NO5) 15 µM HL60 -0.903 1419 Monocrotaline (C16H23NO6) 12 µM PC3 -0.881 7127 LY-294002 (C19H17NO3) 10 µM HL60 -0.869 1168 Aceclofenac (C16H13Cl2NO4) 11 µM PC3 -0.863 7269 Hyoscyamine (C17H23NO3) 14 µM HL60 -0.859 1424 Heliotrine (C16H27NO5) 13 µM HL60 -0.85 2180 Trichostatin A (C17H22N2O3) 1 µM HL60 -0.85 1175 Sulfachlorpyridazine (C10H8ClN4O2S) 14 µM HL60 -0.85 2191 Diflorasone (C22H28F2O5) 8 µM HL60 -0.838 2142 Yohimbine (C21H26N2O3) 10 µM MCF7 -0.833 6777 Sulfadiazine (C10H10N4O2S) 16 µM HL60 -0.833 1852 Pargyline (C11H13N) 20 µM PC3 -0.832 2102 Haloperidol (C21H23ClFNO2) 10 µM HL60 -0.829 6203 Methazolamide (C5H8N4O3S2) 17 -

European Surveillance of Healthcare-Associated Infections in Intensive Care Units
TECHNICAL DOCUMENT European surveillance of healthcare-associated infections in intensive care units HAI-Net ICU protocol Protocol version 1.02 www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT European surveillance of healthcare- associated infections in intensive care units HAI-Net ICU protocol, version 1.02 This technical document of the European Centre for Disease Prevention and Control (ECDC) was coordinated by Carl Suetens. In accordance with the Staff Regulations for Officials and Conditions of Employment of Other Servants of the European Union and the ECDC Independence Policy, ECDC staff members shall not, in the performance of their duties, deal with a matter in which, directly or indirectly, they have any personal interest such as to impair their independence. This is version 1.02 of the HAI-Net ICU protocol. Differences between versions 1.01 (December 2010) and 1.02 are purely editorial. Suggested citation: European Centre for Disease Prevention and Control. European surveillance of healthcare- associated infections in intensive care units – HAI-Net ICU protocol, version 1.02. Stockholm: ECDC; 2015. Stockholm, March 2015 ISBN 978-92-9193-627-4 doi 10.2900/371526 Catalogue number TQ-04-15-186-EN-N © European Centre for Disease Prevention and Control, 2015 Reproduction is authorised, provided the source is acknowledged. TECHNICAL DOCUMENT HAI-Net ICU protocol, version 1.02 Table of contents Abbreviations ............................................................................................................................................... -

Using Saccharomyces Cerevisiae for the Biosynthesis of Tetracycline Antibiotics
Using Saccharomyces cerevisiae for the Biosynthesis of Tetracycline Antibiotics Ehud Herbst Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Ehud Herbst All rights reserved ABSTRACT Using Saccharomyces cerevisiae for the biosynthesis of tetracycline antibiotics Ehud Herbst Developing treatments for antibiotic resistant bacterial infections is among the most urgent public health challenges worldwide. Tetracyclines are one of the most important classes of antibiotics, but like other antibiotics classes, have fallen prey to antibiotic resistance. Key small changes in the tetracycline structure can lead to major and distinct pharmaceutically essential improvements. Thus, the development of new synthetic capabilities has repeatedly been the enabling tool for powerful new tetracyclines that combatted tetracycline-resistance. Traditionally, tetracycline antibiotics were accessed through bacterial natural products or semisynthetic analogs derived from these products or their intermediates. More recently, total synthesis provided an additional route as well. Importantly however, key promising antibiotic candidates remained inaccessible through existing synthetic approaches. Heterologous biosynthesis is tackling the production of medicinally important and structurally intriguing natural products and their unnatural analogs in tractable hosts such as Saccharomyces cerevisiae. Recently, the heterologous biosynthesis of several tetracyclines was achieved in Streptomyces lividans through the expression of their respective biosynthetic pathways. In addition, the heterologous biosynthesis of fungal anhydrotetracyclines was shown in S. cerevisiae. This dissertation describes the use of Saccharomyces cerevisiae towards the biosynthesis of target tetracyclines that have promising prospects as antibiotics based on the established structure- activity relationship of tetracyclines but have been previously synthetically inaccessible. -

Adsorption of Tetracycline by a Tailor- Made Adsorbent in Aqueous System
Journal of Applied Biotechnology & Bioengineering Research Article Open Access Adsorption of tetracycline by a tailor- made adsorbent in aqueous system Abstract Volume 6 Issue 1 - 2019 Tetracyclines are frequently used antibiotics for growth promotion and therapeutic Adelagun Ruth Olubukola Ajoke pharmaceuticals both by humans and animal husbandry, and commonly encountered in Department of Chemical Sciences, Federal University, Nigeria municipal wastewater treatment plants and in the environment in their active form. This implies their continuous release into the environment may facilitate toxic effects both Correspondence: Adelagun Ruth Olubukola Ajoke, on humans and the environment including development of resistance strains, among Department of Chemical Sciences, Federal University, Wukari, others. This research was focused on the synthesis, characterisation and assessment Nigeria, Email of a tailor- made adsorbent: modified chitosan flakes, using several materials for the modification of chitosan to enhance its sorption properties thereby facilitating a higher Received: December 24, 2018 | Published: February 01, 2019 percentage of TC removal from a synthetic pharmaceutical wastewater. TC adsorption onto the modified chitosan flakes was relatively fast (equilibrium time = 2h). Sorption studies revealed that TC removal by the adsorbent followed pseudo second order kinetics and Freundlich isotherm models. At higher TC input concentration, the amount of TC removed was also higher, this implied the sorption was concentration dependent. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies
Arrhythmogenic potential of drugs FP7-HEALTH-241679 http://www.aritmo-project.org/ Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies V 1.3 Draft Lead beneficiary: EMC Date: 03/01/2010 Nature: Report Dissemination level: D5.2 Report on Common Study Protocol for Observational Database Studies WP5: Conduct of Additional Observational Security: Studies. Author(s): Gianluca Trifiro’ (EMC), Giampiero Version: v1.1– 2/85 Mazzaglia (F-SIMG) Draft TABLE OF CONTENTS DOCUMENT INFOOMATION AND HISTORY ...........................................................................4 DEFINITIONS .................................................... ERRORE. IL SEGNALIBRO NON È DEFINITO. ABBREVIATIONS ......................................................................................................................6 1. BACKGROUND .................................................................................................................7 2. STUDY OBJECTIVES................................ ERRORE. IL SEGNALIBRO NON È DEFINITO. 3. METHODS ..........................................................................................................................8 3.1.STUDY DESIGN ....................................................................................................................8 3.2.DATA SOURCES ..................................................................................................................9 3.2.1. IPCI Database .....................................................................................................9 -

Statistical Analysis Plan / Data Specifications the Risk of Acute Liver Injury Associated with the Use of Antibiotics. a Replica
WP6 validation on methods involving an extended audience Statistical analysis plan / data specifications The risk of acute liver injury associated with the use of antibiotics. A replication study in the Utrecht Patient Oriented Database Version: July 11, 2013 Authors: Renate Udo, Marie L De Bruin Reviewers: Work package 6 members (David Irvine, Stephany Tcherny-Lessenot) 1 1. Context The study described in this protocol is performed within the framework of PROTECT (Pharmacoepidemiological Research on Outcomes of Therapeutics by a European ConsorTium). The overall objective of PROTECT is to strengthen the monitoring of the benefit-risk of medicines in Europe. Work package 6 “validation on methods involving an extended audience” aims to test the transferability/feasibility of methods developed in other WPs (in particular WP2 and WP5) in a range of data sources owned or managed by Consortium Partners or members of the Extended Audience. The specific aims of this study within WP6 are: to evaluate the external validity of the study protocol on the risk of acute liver injury associated with the use of antibiotics by replicating the study protocol in another database, to study the impact of case validation on the effect estimate for the association between antibiotic exposure and acute liver injury. Of the selected drug-adverse event pairs selected in PROTECT, this study will concentrate on the association between antibiotic use and acute liver injury. On this topic, two sub-studies are performed: a descriptive/outcome validation study and an association study. The descriptive/outcome validation study has been conducted within the Utrecht Patient Oriented Database (UPOD). -

Critically Important Antimicrobials for Human Medicine – 5Th Revision. Geneva
WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) Critically Important Antimicrobials for Human Medicine 5th Revision 2016 Ranking of medically important antimicrobials for risk management of antimicrobial resistance due to non-human use Critically important antimicrobials for human medicine – 5th rev. ISBN 978-92-4-151222-0 © World Health Organization 2017, Updated in June 2017 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial- ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/ igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. Critically important antimicrobials for human medicine – 5th rev. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.