Variations in Pilosity in a Group of Indonesians Teuku JACOB
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
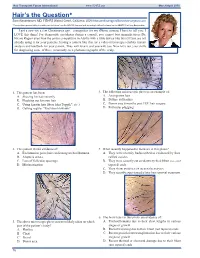
Hair's the Question*
Hair Transplant Forum International www.ISHRS.org March/April 2015 Hair’s the Question* Sara Wasserbauer, MD, FISHRS Walnut Creek, California, USA [email protected] *The questions presented by the author are not taken from the ABHRS item pool and accordingly will not be found on the ABHRS Certifying Examination. I got a new toy a few Christmases ago—a magnifier for my iPhone camera. I have to tell you: I LOVE this thing! For diagnositic usefulness during a consult, you cannot beat magnification (Dr. Nicole Rogers even won the poster competition in Alaska with a little device like this)! If you are not already using it for your patients, having a camera like this (or a video microscope) enables instant analysis and feedback for your patient. They will love it and you will, too. Now let’s test your skills for diagnosing some of these commonly seen photomicrographs of the scalp. 1. This patient has been: 4. The following microscopic photo is an example of: A. Shaving his hair recently A. An ingrown hair B. Plucking out his own hair B. Diffuse folliculitis C. Using keratin hair fibers (aka Toppik®, etc.) C. Donor area 6 months post FUE hair surgery D. Getting regular “Brazilian blowouts” D. Follicular plugging 2. This patient shows evidence of: 5. What recently happened to the hairs in this photo? A. Exclamation point hairs indicating trichotillomania A. They were recently backcombed as evidenced by their B. Alopecia areata ruffled cuticle. C. Loss of follicular openings B. They were recently cut as shown by their blunt (i.e., not D. -

Temporary Hair Removal by Low Fluence Photoepilation: Histological Study on Biopsies and Cultured Human Hair Follicles
Lasers in Surgery and Medicine 40:520–528 (2008) Temporary Hair Removal by Low Fluence Photoepilation: Histological Study on Biopsies and Cultured Human Hair Follicles 1 2 3 4 Guido F. Roosen, MSc, Gillian E. Westgate, PhD, Mike Philpott, PhD, Paul J.M. Berretty, MD, PhD, 5 6 Tom (A.M.) Nuijs, PhD, * and Peter Bjerring, MD, PhD 1Philips Electronics Nederland, 1077 XV Amsterdam, The Netherlands 2Westgate Consultancy Ltd., Bedford MK 43 7QT, UK 3Queen Mary’s School of Medicine and Dentistry, London E1 2AT, UK 4Catharina Hospital, 5602 ZA Eindhoven, The Netherlands 5Philips Research, 5656 AE Eindhoven, The Netherlands 6Molholm Hospital, DK-7100 Vejle, Denmark Background and Objectives: We have recently shown INTRODUCTION that repeated low fluence photoepilation (LFP) with Clinical results of photoepilation treatments reported in intense pulsed light (IPL) leads to effective hair removal, the literature in general show variability in hair reduction which is fully reversible. Contrary to permanent hair effectiveness, both in rate and duration of clearance. Based removal treatments, LFP does not induce severe damage to on ‘‘selective photothermolysis’’ as the proposed mecha- the hair follicle. The purpose of the current study is to nism of action [1], this variability can partly be explained by investigate the impact of LFP on the structure and the the broad range of applied parameters such as fluence, physiology of the hair follicle. pulse width and spectrum of the light. Similarly, variability Study Design/Materials and Methods: Single pulses of between subjects such as skin type, hair color, and hair 2 IPL with a fluence of 9 J/cm and duration of 15 milliseconds follicle (HF) geometry also contributes to these differences were applied to one lower leg of 12 female subjects, followed [2–4]. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Neuromechanics of Maneuverability: Sensory-Neural and Mechanical Processing for the Control of High-Speed Locomotion Permalink https://escholarship.org/uc/item/1b79g7xw Author Mongeau, Jean-Michel Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Neuromechanics of Maneuverability: Sensory-Neural and Mechanical Processing for the Control of High-Speed Locomotion By Jean-Michel Mongeau A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Biophysics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Robert J. Full, Chair Professor Noah J. Cowan Professor Ronald S. Fearing Professor Frederic E. Theunissen Spring 2013 Neuromechanics of Maneuverability: Sensory-Neural and Mechanical Processing for the Control of High-Speed Locomotion 2013 by Jean-Michel Mongeau Abstract Neuromechanics of Maneuverability: Sensory-Neural and Mechanical Processing for the Control of High-Speed Locomotion by Jean-Michel Mongeau Doctor of Philosophy in Biophysics University of California, Berkeley Professor Robert J. Full, Chair Maneuverability in animals is unparalleled when compared to the most maneuverable human- engineered mobile robot. Maneuverability arises in part from animals’ ability to integrate multimodal sensory information with an ongoing motor program while interacting within a spatiotemporally -

(In)Determinable: Race in Brazil and the United States
Michigan Journal of Race and Law Volume 14 2009 Determining the (In)Determinable: Race in Brazil and the United States D. Wendy Greene Cumberland School fo Law at Samford University Follow this and additional works at: https://repository.law.umich.edu/mjrl Part of the Comparative and Foreign Law Commons, Education Law Commons, Law and Race Commons, and the Law and Society Commons Recommended Citation D. W. Greene, Determining the (In)Determinable: Race in Brazil and the United States, 14 MICH. J. RACE & L. 143 (2009). Available at: https://repository.law.umich.edu/mjrl/vol14/iss2/1 This Article is brought to you for free and open access by the Journals at University of Michigan Law School Scholarship Repository. It has been accepted for inclusion in Michigan Journal of Race and Law by an authorized editor of University of Michigan Law School Scholarship Repository. For more information, please contact [email protected]. DETERMINING THE (IN)DETERMINABLE: RACE IN BRAZIL AND THE UNITED STATES D. Wendy Greene* In recent years, the Brazilian states of Rio de Janeiro, So Paulo, and Mato Grasso du Sol have implemented race-conscious affirmative action programs in higher education. These states established admissions quotas in public universities '' for Afro-Brazilians or afrodescendentes. As a result, determining who is "Black has become a complex yet important undertaking in Brazil. Scholars and the general public alike have claimed that the determination of Blackness in Brazil is different than in the United States; determining Blackness in the United States is allegedly a simpler task than in Brazil. In Brazil it is widely acknowledged that most Brazilians are descendants of Aficans in light of the pervasive miscegenation that occurred during and after the Portuguese and Brazilian enslavement of * Assistant Professor of Law, Cumberland School of Law at Samford University. -

The Hairlessness Norm Extended: Reasons for and Predictors of Women’S Body Hair Removal at Different Body Sites
Sex Roles (2008) 59:889–897 DOI 10.1007/s11199-008-9494-3 ORIGINAL ARTICLE The Hairlessness Norm Extended: Reasons for and Predictors of Women’s Body Hair Removal at Different Body Sites Marika Tiggemann & Suzanna Hodgson Published online: 18 June 2008 # Springer Science + Business Media, LLC 2008 Abstract The study aimed to explore the motivations prescription renders many women not only perpetually behind and predictors of the practice of body hair removal dissatisfied with their bodies (Rodin et al. 1985), but also among women. A sample of 235 Australian female highly motivated to alter their bodies to match the ideal, as undergraduate students completed questionnaires asking illustrated by the existence of multi-million dollar diet, about the frequency and reasons for body hair removal, as exercise, cosmetic and cosmetic surgery industries. well as measures of media exposure. It was confirmed that One particular aspect of the ideal that has received the vast majority (approximately 96%) regularly remove relatively little research attention or theorizing is the their leg and underarm hair, most frequently by shaving, prescription for smooth hairless skin. This is most likely and attribute this to femininity and attractiveness reasons. A because the practice of removing unwanted body hair is so sizeable proportion (60%) also removed at least some of normative in Western cultures as to go unremarked. By far their pubic hair, with 48% removing most or all of it. Here the majority of women in the USA (Basow 1991), UK the attributions were relatively more to sexual attractiveness (Toerien et al. 2005) and Australia (Tiggemann and Kenyon and self-enhancement. -

Open Pritchard Dissertation 2018
The Pennsylvania State University The Graduate School Department of Ecosystem Science and Management A LANDSCAPE OF FEAR: MEASURING NUTRITIONAL AND PSYCHOLOGICAL STRESS IN PREDATOR-PREY INTERACTIONS A Dissertation in Wildlife and Fisheries Science by Catharine Pritchard © 2018 Catharine Pritchard Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2018 The dissertation of Catharine Pritchard was reviewed and approved* by the following: Tracy Langkilde Professor and Head of Biology, The Pennsylvania State University Dissertation Advisor C. Paola Ferreri Associate Professor of Fisheries Management, The Pennsylvania State University Chair of Committee Victoria Braithwaite Professor, The Pennsylvania State University Matthew Marshall Adjunct Assistant Professor of Wildlife Conservation, The Pennsylvania State University Michael Messina Professor and Head of Ecosystem Science and Management, The Pennsylvania State University *Signatures are on file in the Graduate School iii ABSTRACT Animals commonly respond to stimuli, including risk of predation, nutritional deficits, and disturbance through the stress response. The most commonly measured stress hormones, glucocorticoids (GCs), then generally become elevated, energy is diverted away from non- essential processes, and behavior is modified to facilitate short-term survival. Because GCs can be collected noninvasively, they are candidates for evaluating health in wild animals. However, few studies have tested critical assumptions about GCs and -

Hair Removal & Skin Rejuvenation Machine
Hair Removal & Skin Rejuvenation Machine IPL Hair Removal IPL laser hair removal is a very popular method of hair removal. An IPL laser uses “intense pulsed light” to gently and safely removes hair on the face, legs, back, and bikini/pubic areas. The pulses of light focus their heat on the hair follicles, destroying them without burning or damaging the skin. IPL laser hair removal is quick and veritably painless. Side effects are few, but may include redness or swelling of the treated area; this should disappear rapidly. Good candidates for IPL laser hair removal will have lighter skin and darker hair, since IPL targets pigment in the hair follicles. Many people have experienced permanent hair removal with this method; if hair does grow back, it is often sparse and finer in texture. Laser Hair removal. Laser hair removal is a gentle and effective way to permanently reduce unwanted hair from virtually any area of the body. Locate a laser hair removal clinic in your area and acquire information on the latest laser hair removal options for men and women by reading the sections below. Laser Hair Removal Procedure Laser hair removal is most effective when hair is in the growth stage. Because hair grows in cycles, laser hair removal patients typically require a series of four to six sessions spaced approximately one month apart for maximum results. www.globalclinic.co During Laser Hair Removal Treatment Typically, the areas to be treated are shaved a few days prior to the laser hair removal treatment. On the day of the procedure, an anesthetic cream may be applied, although this step is not necessary. -

Objective Comparison of Shampoo Bars, Natural and Synthetic by Kerri Mixon Shampoo Bars and Synthetic Shampoos Have Different Effects on Hair
Objective Comparison of Shampoo Bars, Natural and Synthetic By Kerri Mixon Shampoo bars and synthetic shampoos have different effects on hair. Learn which ingredients are commonly added to cold process soap to make shampoo bars. Understand the chemical and physical effects of natural and synthetic shampoos, how the effects vary by ethnic hair structure, and the added benefits of using a conditioner. This presentation focuses on hard scientific facts, not subjective perspectives or opinions. As a 16th generation professional soapmaker for 20 years, I thought I had shampoo bar soaps down cold. I thoughtfully formulated my shampoo soaps with 10–15% castor oil to yield a thicker, longer-lasting lather and chose herbal infusions beneficial to hair, such as henna, chamomile, sage, horsetail, and rosemary. I’d always heard hair products needed to have a lower pH, so I included small amounts of organic apple cider vinegar (which is acidic) to help lower the pH of my shampoo soaps and I included small amounts of citric acid (also acidic) to act as a mild chelating agent and pH buffer. My customers seem to enjoy my shampoo soap bars and regularly re-order my shampoo soap bars and my liquid soap shampoo, so I thought all was well. I know Lush and other companies make synthetic detergent bars marketed as solid shampoos, but detergents aren’t my thing—and I know my customers don’t want anything to do with synthetic detergents! I have a strong background in biology and chemistry, so when Leigh O’Donnell asked me to prepare a comparison of soap shampoo bars versus detergent shampoo bars, I jumped at the chance to prove soap as the superior champion, once and for all. -

Puberty Changes in Boys
PUBERTY CHANGES IN BOYS The changes of puberty in boys include growth of testes, growth of the penis, growth of pubic hair, voice change, and height spurt. An individual boy may begin his changes at a time different from his peers. TESTES DEVELOPMENT Growth of testes is usually the first change for boys. The scrotum lengthens and becomes a darker color. These changes begin between 9 ½ and 13 ½ years of age. PENIS DEVELOPMENT Growth of the penis occurs between ages 10 and 14. PUBIC HAIR Pubic hair and underarm hair appear between the ages of 10 and 14. At first the hair is fine and thin but becomes curly and coarse over time. GROWTH SPURT Increased height and change in body shape with broadening shoulders begin between the ages of 11 and 16. The voice also deepens. During this time, a boy may notice some temporary breast enlargement. This is completely normal, very common, and is no cause for concern. It will disappear with time. FACIAL AND CHEST HAIR Facial and chest hair appear at around age 17. The thickness of the beard is quite variable and depends on genes. Some men have little or no chest hair and very light beards, while others have dense growth. The amount of hair is unrelated to masculinity. ERECTIONS / EJACULATIONS Erections occur more often during puberty; sometimes a boy will have an erection for no apparent reason – this is common and normal. Ejaculation, the release of semen from the penis, begins during puberty. Like erections, ejaculation can occur for no obvious reason, especially during sleep. -

Does Materialism Predict Body Hair Removal Among Undergraduate Males and Females? Brianna Ballew Grand Valley State University, [email protected]
Grand Valley State University ScholarWorks@GVSU Honors Projects Undergraduate Research and Creative Practice 4-2016 Does Materialism Predict Body Hair Removal Among Undergraduate Males and Females? Brianna Ballew Grand Valley State University, [email protected] Follow this and additional works at: http://scholarworks.gvsu.edu/honorsprojects Part of the Arts and Humanities Commons Recommended Citation Ballew, Brianna, "Does Materialism Predict Body Hair Removal Among Undergraduate Males and Females?" (2016). Honors Projects. 576. http://scholarworks.gvsu.edu/honorsprojects/576 This Open Access is brought to you for free and open access by the Undergraduate Research and Creative Practice at ScholarWorks@GVSU. It has been accepted for inclusion in Honors Projects by an authorized administrator of ScholarWorks@GVSU. For more information, please contact [email protected]. Materialist Values and Body Hair Removal 1 Running head: Materialist Values and Body Hair Removal Does Materialism Predict Body Hair Removal Among Undergraduate Males and Females? Brianna Ballew Grand Valley State University Faculty Advisor: Donna Henderson-King Ph.D. – Psychology Department 499 Honors Senior Project April, 2016 Materialist Values and Body Hair Removal 2 Abstract Previous research indicates that materialistic women are more likely to want to alter their bodies (Henderson-King & Brooks, 2009). This study focuses specifically on the relationship between materialism and body hair removal. We collected information about the frequency of body hair removal, reasons for hair removal, and materialism (Richins & Dawson, 1992). Findings indicate that males and females do not significantly differ on materialist values. Correlational analyses reveal that for women, materialism is related to frequency of hair removal for several body sites; for men, however, materialism was related to body hair removal for only a single site. -
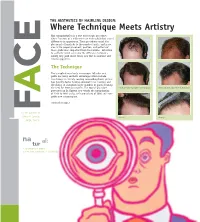
Where Technique Meets Artistry Hair Transplantation Is a True Microscopic Procedure, Where Fractions of a Millimeter Can Make Subtle but Critical
THE AESTHETICS OF HAIRLINE DESIGN: Where Technique Meets Artistry Hair transplantation is a true microscopic procedure, where fractions of a millimeter can make subtle but critical E differences in appearance. These procedures entail the placement of hundreds to thousands of grafts, and in no area is the proper placement, position, and pattern of these grafts more important than the hairline. Attention to aesthetic detail can make the difference between a merely very good result versus one that is excellent and natural appearing. C The Technique The transplanting of only microscopic follicular unit grafts has many aesthetic advantages which include: less damage to already existing surrounding hairs; greater hair density; faster healing; minimal to no scarring; and the ability to transplant larger numbers of grafts meaning the need for fewer procedures. The typical procedure Before and after a procedure of 2400 grafts Before and after a procedure of 2500 grafts performed by Dr. Epstein now entails the transplanting A of 2200 to 2400 grafts, with procedures of 3000 and more grafts now commonplace. continued on page 2 F For the patients of Jeffrey S. Epstein, Close-up Close-up M. D., F.A .C.S. na al: tur 1. As existing in nature 2. Free from pretension or artificiality WORDS FROM THE DOCTOR THE AESTHETICS OF HAIRLINE DESIGN (cont.) Since 1993, I have been fortunate to participate in the evolution The Aesthetics of the specialties of surgical hair The transplanted hairlines on this page all restoration and facial plastic demonstrate different aesthetic aspects of a natural a surgery. Few other surgical appearing hairline. -

Etiology and Treatment of Hirsutism
Central Journal of Endocrinology, Diabetes & Obesity Bringing Excellence in Open Access Review Article *Corresponding author Maria Palmetun Ekbäck, Pharmacology and Therapeutic Department, Örebro Region County Etiology and Treatment of Council, Dermatology Department, Örebro University Hospital, Örebro, Sweden, Email: maria.palmetun- Hirsutism [email protected] Submitted: 28 August 2017 Maria Palmetun Ekbäck* Accepted: 31 August 2017 Pharmacology and Therapeutic Department, Örebro Region County Council, Dermatology Published: 31 August 2017 Department, Örebro University Hospital, Örebro, Sweden ISSN: 2333-6692 Copyright Abstract © 2017 Ekbäck Hirsutism, excessive hair growth in women in a male pattern distribution, is an international OPEN ACCESS issue and approximately 5 to 15% of the general population of women is reported to be hirsute. It causes profound stress in women. As hirsutism is a symptom and not a disease it is Keywords important to find the underlying cause. Polycystic ovary syndrome is the most common cause but • Hirsutism other not so common endocrinology disorders must be excluded. Mild hirsutism could be seen • PCOS in a woman with normal menses and normal androgen levels (idiopathic hirsutism). Ferriman- • Impaired QoL Gallwey scale (F-G) is used for assessment of hairiness. The maximum score is 36 and a score • Ferriman-Gallwey scale over 8 is considered as a hirsuid state. The aim of the medical treatment is to correct the • Medical treatment hormonal imbalance and stop further progress. Oral contraceptives (OCP) are recommended • Photo-epilation as first line treatment. Spironolactone is the first choice if there is indication for antiandrogen therapy. Antiandrogens should be combined with an OCP as antiandrogens are teratogenic. Photo-epilation or electrolysis is mostly needed in order to reduce the amount of hair.