Drawing Sugar Structures: Fischer Projections, Haworth Structures and Chair Conformers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MM# Modeling of Aldopentose Pyranose Rings Michael K
Chemical and Biological Engineering Publications Chemical and Biological Engineering 2002 MM# Modeling of Aldopentose Pyranose Rings Michael K. Dowd United States Department of Agriculture William M. Rockey Iowa State University Alfred D. French United States Department of Agriculture See next page for additional authors Follow this and additional works at: http://lib.dr.iastate.edu/cbe_pubs Part of the Biochemical and Biomolecular Engineering Commons, and the Biological Engineering Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ cbe_pubs/31. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemical and Biological Engineering at Iowa State University Digital Repository. It has been accepted for inclusion in Chemical and Biological Engineering Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. MM# Modeling of Aldopentose Pyranose Rings Abstract MM3 (version 1992, ϵ=3.0) was used to study the ring conformations of d-xylopyranose, d-lyxopyranose and d-arabinopyranose. The nee rgy surfaces exhibit low-energy regions corresponding to chair and skew forms with high-energy barriers between these regions corresponding to envelope and half-chair forms. The lowest 4 energy conformer is C 1 for α- and β-xylopyranose and α- and β-lyxopyranose, and the lowest energy 1 conformer is C 4 for α- and β-arabinopyranose. Only α-lyxopyranose exhibits a secondary low-energy region 1 ( C 4) within 1 kcal/mol of its global minimum. -

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Low Fructose Diet
Low Fructose Diet What is Fructose? Fructose is a naturally occurring simple sugar found in fruit, vegetables, and honey. Fructose intolerance can occur in people with irritable bowel syndrome and other GI disorders. Fruits and fruit juices with higher levels of fructose may cause gas, bloating, abdominal cramping, and diarrhea. Glucose is also a naturally occurring sugar. The more glucose than fructose in a product, the more “intestinal friendly” the fruit or fruit juice may be. High Fructose Corn Syrup (HFCS) **** HFCS is made up of almost half glucose and half fructose and may be absorbed just as well as sucrose (regular table sugar). Therefore, items with HFCS such as soft drinks, may be tolerated well when limited to 12 oz per day and with a meal. HFCS can also be found in canned, baked, or processed foods. **** In some patients, even a small amount of processed fruit juice or even foods with HFCS may cause as much malabsorption, and/or intestinal discomfort, as eating large quantities of fruit. Sorbitol Sorbitol or Sorbose is a sugar alcohol used as an artificial sweetener and found naturally in fruits and fruit juices. It can also be found in many “diet foods” such as diet soft drinks, sugarless gum, sugar-free jelly/jam, and liquid medications. Sorbitol often creates similar symptoms as fructose – especially when fructose and sorbitol are ingested together. General Guidelines • Eliminate products with ingredients that list fructose, crystalline fructose (not HFCS), honey, and sorbitol on the label. • Avoid sugar alcohols which include sorbitol, isomalt, lactitol, maltitol, mannitol, xylitol, erythrytol, and lactatol. -
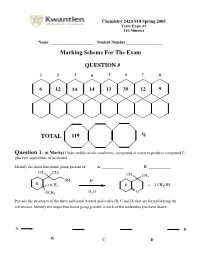
Marking Scheme for the Exam
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Evidence for Two Asymmetric Conformational States in the Human Erythrocyte Sugar-Transport System by JOHN E
Biochem. J. (1975) 145, 417429 417 Printed in Great Britain Evidence for Two Asymmetric Conformational States in the Human Erythrocyte Sugar-Transport System By JOHN E. G. BARNETT, GEOFFREY D. HOLMAN, R. ALAN CHALKLEY and KENNETH A. MUNDAY Department ofPhysiology and Biochlemistry, University ofSouthampton, Southampton S09 3TU, U.K. (Received 4 June 1974) 6-0-Methyl-, 6-0-propyl-, 6-0-pentyl- and 6-0-benzyl-D-galactose, and 6-0-methyl-, 6-0-propyl- and 6-0-pentyl-D-glucose inhibit the glucose-transport system of the human erythrocyte when added to the external medium. Penetration of 6-0-methyl-D-galactose is inhibited by D-glucose, suggesting that it is transported by the glucose-transport system, but the longer-chain 6-0-alkyl-D-galactoses penetrate by a slower D-glucose- insensitive route at rates proportional to their olive oil/water partition coefficients. 6-0-n-Propyl-D-glucose and 6-0-n-propyl-D-galactose do not significantly inhibit L-sorbose entry or D-glucose exit when present only on the inside of the cells whereas propyl-f6-D-glucopyranoside, which also penetrates the membrane slowly by a glucose- insensitive route, only inhibits L-sorbose entry or D-glucose exit when present inside the cells, and not when on the outside. The 6-0-alkyl-D-galactoses, like the other non- transported C4 and C-6 derivatives, maltose and 4,6-0-ethylidene-D-glucose, protect against fluorodinitrobenzene inactivation, whereas propyl ,B-D-glucopyranoside stimulates the inactivation. Of the transported sugars tested, those modified at C-1, C-2 and C-3 enhance fluorodinitrobenzene inactivation, where those modified at C-4 and C-6 do not, but are inert or protect against inactivation. -

Structural Features
1 Structural features As defined by the International Union of Pure and Applied Chemistry gly- cans are structures of multiple monosaccharides linked through glycosidic bonds. The terms sugar and saccharide are synonyms, depending on your preference for Arabic (“sukkar”) or Greek (“sakkēaron”). Saccharide is the root for monosaccha- rides (a single carbohydrate unit), oligosaccharides (3 to 20 units) and polysac- charides (large polymers of more than 20 units). Carbohydrates follow the basic formula (CH2O)N>2. Glycolaldehyde (CH2O)2 would be the simplest member of the family if molecules of two C-atoms were not excluded from the biochemical repertoire. Glycolaldehyde has been found in space in cosmic dust surrounding star-forming regions of the Milky Way galaxy. Glycolaldehyde is a precursor of several organic molecules. For example, reaction of glycolaldehyde with propenal, another interstellar molecule, yields ribose, a carbohydrate that is also the backbone of nucleic acids. Figure 1 – The Rho Ophiuchi star-forming region is shown in infrared light as captured by NASA’s Wide-field Infrared Explorer. Glycolaldehyde was identified in the gas surrounding the star-forming region IRAS 16293-2422, which is is the red object in the centre of the marked square. This star-forming region is 26’000 light-years away from Earth. Glycolaldehyde can react with propenal to form ribose. Image source: www.eso.org/public/images/eso1234a/ Beginning the count at three carbon atoms, glyceraldehyde and dihydroxy- acetone share the common chemical formula (CH2O)3 and represent the smallest carbohydrates. As their names imply, glyceraldehyde has an aldehyde group (at C1) and dihydoxyacetone a carbonyl group (at C2). -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

The Food Lawyers® Respectfully Request That FDA Implements the Following
December 7, 2020 Dockets Management Staff (HFA-305) Filed Electronically Food and Drug Administration https://www.regulations.gov Re: Sugars Metabolized Differently than Traditional Sugars (FDA-2020-N-1359) Ladies and Gentlemen: One Page Executive Summary FDA’s seeks information to “… promote the public health and help consumers make informed dietary decisions” regarding sugars that are metabolized differently than traditional sugars. Given the nation’s battles with diabetes and obesity, and the benefits that non-traditional sugars can offer in these battles, the Agency’s stated public policy goal goes to the very heart of American consumers’ health. This laudatory public policy’s realization is complicated by a lack of consumer awareness of how some sugars are metabolized differently than others. In an effort to answer the questions posed by the Agency regarding the treatment of Sugars that Are Metabolized Differently Than Traditional Sugars, we suggest that the Agency adapt a mechanism that will seek to harmonize the public policy of promoting public health with consumers’ lack of awareness of sugars that are metabolized differently than sucrose. In particular, we suggest that FDA should consider the following: 1. Establish a new category of sugars called Rare Sugars that exhibit the following characteristics: a. Are naturally occurring b. Impart a sweet taste that is at least 50% the sweetness of sucrose c. 2.0 kcal/g or less. d. Resulting pH of 6.0 or greater of dental plaque after consumption. e. No or low glycemic response. f. No or low insulinemic response. 2. Exclude Rare Sugars from “Total Sugars” and “Added Sugars” declarations to stimulate their deployment by industry and consumption by the public. -

WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23G 4/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 12/062043 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 26 October 2012 (26.10.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/556,546 7 November 20 11 (07. 11.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): WVI. kind of regional protection available): ARIPO (BW, GH, WRIGLEY JR. COMPANY [US/US]; 1132 Blackhawk GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, Street, Chicago, IL 60642 (US). -

Fall 2014� HO OH O OH O OH
Fall 2014! HO OH O OH O OH HO OH HO OH !-D-ribofuranose (Haworth) HO HO O O HO OH OH HO HO OH !-D-mannopyranose HO OH (Haworth) anomeric anomeric carbon carbon The anomeric monosaccharides, α-D-glucopyranose and β-D-glucopyranose, drawn as Fischer and Haworth projections, and as ball-and-stick models Upon cyclization, the carbonyl carbon becomes chiral and is referred to as the anomeric carbon. In the α-form, the anomeric OH (O1) is on the opposite side of the ring from the CH2OH group, and in the β-form, O1 is on the same side. The α- and β-forms are referred to as anomers or anomeric pairs, and they interconvert in aqueous solution via the acyclic (“linear”) form (anomerization). Aqueous solutions of D-glucose contain ~64% β-pyranose and ~36% α-pyranose. OH OH O HO HO H O HO OH OH CHO OH OH !-D-glucopyranose OH OH 37.6 % !-D-glucofuranose HO 0.11 % OH OH OH OH OH O HO H HO D-glucose O OH HO OH acyclic aldehyde OH 0.006 % OH "-D-glucopyranose OH 62.0 % "-D-glucofuranose -H2O +H2O 0.28 % CH(OH)2 OH HO OH OH OH D-glucose acyclic hydrate 0.004 % Monosaccharides that are capable of assuming a form in solution that contains a free carbonyl group can be oxidized by relatively mild oxidizing agents such as Fe+3 or Cu+2 (Fehling’s reaction). The saccharide is oxidized and the reagent is reduced.! CHO COO- OH OH HO cyclic and hydrate HO forms of D-glucose OH OH 2Cu+2 2Cu+ OH OH OH OH D-glucose D-gluconate acyclic aldehyde q " Phosphorylation can restrict the types of cyclization reactions of the acyclic carbonyl form. -

Furanosic Forms of Sugars: Conformational Equilibrium of Methyl B-D-Ribofuranoside† Cite This: Chem
ChemComm View Article Online COMMUNICATION View Journal | View Issue Furanosic forms of sugars: conformational equilibrium of methyl b-D-ribofuranoside† Cite this: Chem. Commun., 2016, 52,6241 Patricia E´cija,a Iciar Uriarte,a Lorenzo Spada,ab Benjamin G. Davis,c Received 5th February 2016, Walther Caminati,b Francisco J. Basterretxea,a Alberto Lesarri*d and Accepted 1st April 2016 Emilio J. Cocinero*a DOI: 10.1039/c6cc01180b www.rsc.org/chemcomm The investigation of an isolated ribofuranose unit in the gas phase reveals the intrinsic conformational landscape of the biologically active sugar form. We report the rotational spectra of two conformers of methyl b-D-ribofuranoside in a supersonic jet expansion. Both 3 conformers adopt a near twisted ( T2) ring conformation with the methoxy and hydroxymethyl substituents involved in various intra- molecular hydrogen bonds. Scheme 1 The pyranose (1) and furanose (2) constitutional isomers of ribose, together with methyl-b-D-ribofuranoside (3), in Haworth projection. Sugars are flexible polymorphic species, exhibiting complex con- stitutional, configurational and conformational isomerism. The intramolecular reaction between carbonyl (typically reducing (RNA), as substrates (ATP or sugar-diphospho-nucleosides), or as terminus) and hydroxyl groups gives rise to cyclic hemiacetal/ cofactors (NAD(P) or NAD(P)H).7 Remarkably, their roles are often ketals, particularly stable for five- or six-membered ring forms critical: DNA analogues in which thefuranoseringsareexchanged (furanose or pyranose, respectively, Scheme 1). Large amplitude by pyranoses produce double helices with much stronger base motions, like ring puckering, inversion or pseudorotation, com- pairing, but are unsuitable to replace DNA.8 The biochemical bine with the internal rotation of the hydroxyl groups to produce a functionality in ribose-based biomolecules probably relies on Published on 01 April 2016. -

Monosaccharides: a Tof-SIMS Reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, Spectra Database
Monosaccharides: A ToF-SIMS reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, spectra database. II. Positive polarity 01599, 01600, 01601, 01602, a) 01603, 01604, 01605, 01606, Laetitia Bernard, Rowena Crockett, and Maciej Kawecki 01607, 01608, 01609, 01610 Laboratory of Nanoscale Materials Science, Empa, CH-8600 Dübendorf, Switzerland Technique: SIMS (Received 20 August 2019; accepted 30 October 2019; published 3 December 2019) Host Material: Silicon (100) wafer The number of time-of-flight secondary ion mass spectrometry studies on biological tissues and Instrument: IONTOF TOF-SIMS.5 cells has strongly increased since the development of primary ion sources that allow not only ele- Major Species in Spectra: C, H, O, (N) mental but also molecular analysis. Substantial fragmentation during ionic bombardment results in a Minor Species in Spectra: Na, K large number of peaks, rendering data analysis complex. Complete and trustable sets of reference spectra for the main biological building blocks, i.e., amino acids, monosaccharides, fatty acids, and Published Spectra: 19 nucleotides, are required. This work aims to provide an accurate and extensive library of reference Spectra in Electronic Record: 19 + spectra for monosaccharides, measured with the Bi3 primary ion. Here (Paper II), the positive polar- Published Figures: 20 ity spectra and lists of associated characteristic fragments are presented. Published by the AVS. Spectral Category: Reference https://doi.org/10.1116/1.5125103 Keywords: ToF-SIMS, carbohydrate, sugar, monosaccharide, mass spectrometry, fragmentation INTRODUCTION Fluka and all others from Sigma Aldrich. Each powder was dissolved in freshly de-ionized H2O (resistivity >18.2 MΩ cm) Monosaccharides are the building blocks of structural polymers at a concentration of 0.1M.